Section 1 1 Biological molecules and Section 3 6 ending enzymes
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
About Monosaccarides
Sweet-tasting
Soluble
Examples: Glucose, Galactose, fructose
General formula: (CH2O)n, where n = 3 to 7
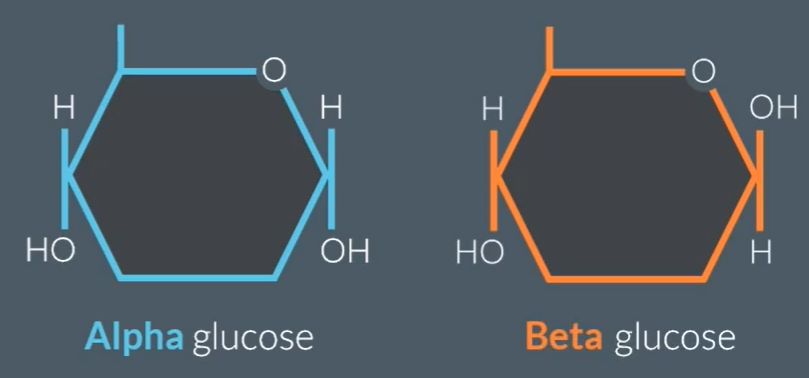
Alpha glucose (above) Beta glucose (hydrogen below)
About Disaccarides
Soluble in water
Often tastes sweet
Examples:
Glucose + Glucose = Maltose
Glucose + Fructose = Sucrose
Glucose + Galactose = Lactose
Formed via condensation reaction when a molecule of water is removed
About Polysaccarides
Insoluble and non-sweet tasting
Example: Starch
Test for reducing and non-reducing sugar
All monosaccarides are reducing sugars.
If reducing sugar present:
Crush to form solution
Add same volume of benedicts regent
Heat above 80 degrees
Colour change to brick red = +
If non-reducing sugar:
Crush to form solution
Add acid
Boil
Neutralize with alkaline (Benedicts regent doesn’t work in alkaline conditions)
Heat
Add benedicts regent
To test for starch
Iodine test
Crush the food sample
Add water
add iodine
If turns blue-black starch present
Different types of polysaccarides:
Cellulose has straight chains, (1-4 glycosidic bonds, alternating glycosidic bonds) Allows strength and rigidity for cell. They form hydrogen bonds between straight chains. They are grouped to form micro fibrils, to enhance strength.
Starch and glycogen: Branched and coiled (1-4, 1-6) Storage molecules, compact due to their coiled structure, cannot cross cell membranes, due to their large size, don’t effect cell water potential as they are insoluble. Used in respiration, they have many end-branches which hydrolysing enzymes can make use of.
Difference between Starch and Glycogen: Glycogen is more heavily branched, so more rapidly hydrolysed.
About lipids: what elements do lipids consist of, their solubility, the main groups
Contain carbon, hydrogen and oxygen
insoluble in water
soluble in organic solvents (alcohols and acetone)
Two main groups of lipids: triglycerides and phospholipids
Roles of lipids
Cell membrane: contribute to the flexibility and transporting molecules across
Source of energy: when oxidised lipids provide twice the amount of energy as the same mass of carbohydrates
Waterproofing: lipids are insoluble (found on waxy cuticle)
Protection: Stored around delicate organs such as kidneys
Insulation: Slow conductors of heat, help retain body heat
Test for lipids
2 cm3 of solution
5 cm3 of ethanol + SHAKE
5 cm3 of water
Cloudy white emulsification = lipids present
KEY: Ethanol THEN water [E comes before W]
Structure of proteins
There is NH2, which is the amine group. The COOH is the carboxylic group.
Primary Structure: sequence of amino acids
Secondary structure: The [C=O]- and [N-H]+ causes attraction between each other, known as hydrogen bonding. This causes the molecule to fold into an alpha helix or a beta pleated sheet.
Tertiary structure: Responsible for the 3D structure of the protein. The forces between R-groups (ionic, disulfide and hydrogen bonding) causes molecule to bend
Quaternary structure: More than one polypeptide bonded together with the same R-groups
![<ul><li><p>There is NH<sub>2</sub>, which is the amine group. The COOH is the carboxylic group.</p></li><li><p>Primary Structure: sequence of amino acids</p></li><li><p>Secondary structure: The [C=O]<sup>-</sup> and [N-H]<sup>+</sup> causes attraction between each other, known as hydrogen bonding. This causes the molecule to fold into an alpha helix or a beta pleated sheet.</p></li><li><p>Tertiary structure: Responsible for the 3D structure of the protein. The forces between R-groups (ionic, disulfide and hydrogen bonding) causes molecule to bend</p></li><li><p>Quaternary structure: More than one polypeptide bonded together with the same R-groups</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/71dad238-67f8-4702-8699-fbedf067e896.png)
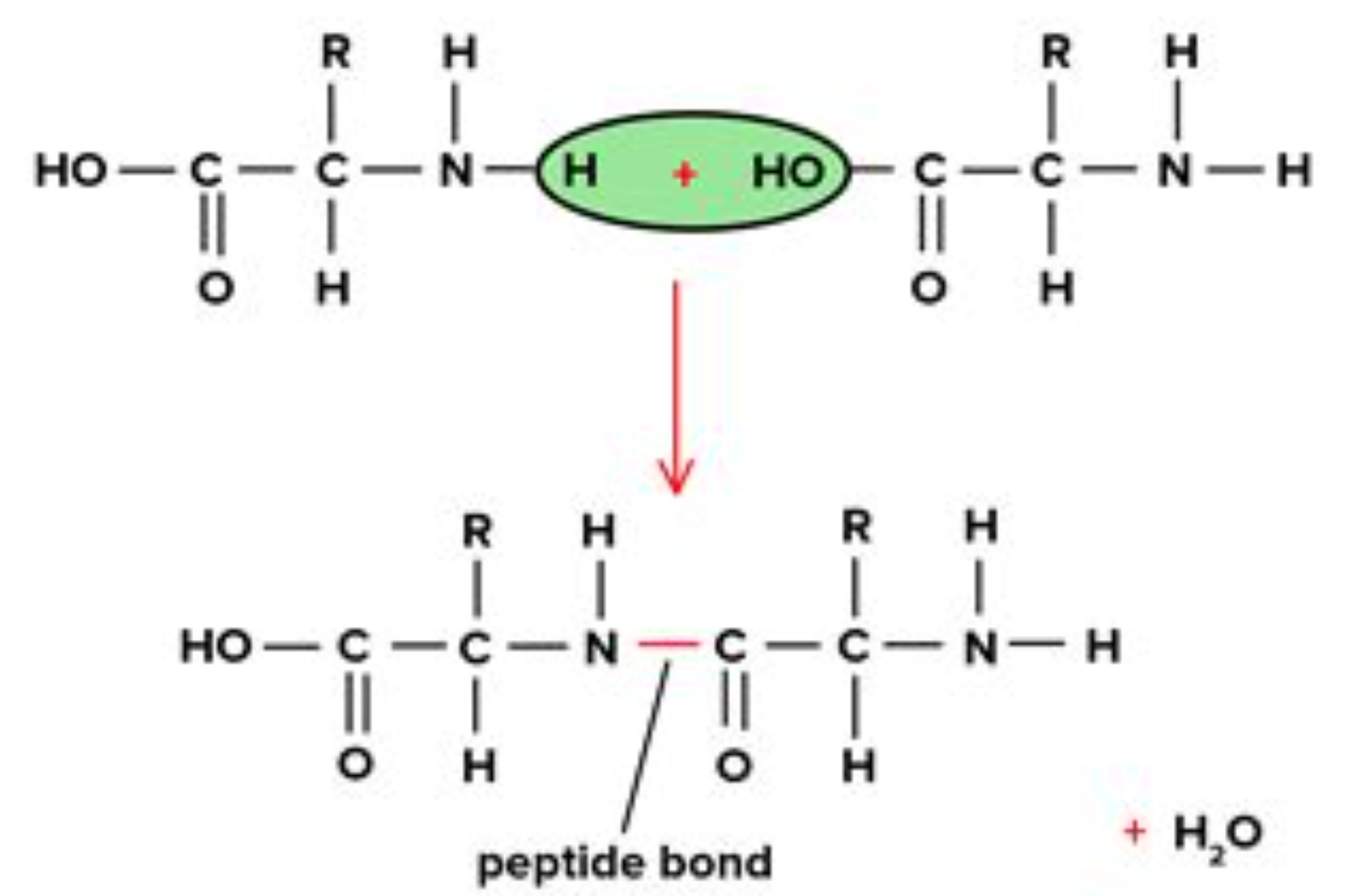
How two amino acids join together?
The OH from the carboxylic group and the H from the amine group join together to form water.
And a peptide bond is formed between the carbon in carboxylic group and nitrate in amine group.
Test for proteins
2 cm3 of food sample
2 cm3 of Biuret regent
Blue to purple protein is present
Saturated Lipid and Unsaturated Lipids
Saturated: No double bonds between the carbon atoms, so they can be more compact in room temperature (solids)
Unsaturated: One or more double bonds between carbon atoms, so they are less compact at room temperatures (liquids // oils)
Role of enzyme
They are biological catalysts that lower the activation energy, they take part in the reaction without being used up themselves.
How is the shape of enzyme determined?
The active site is determined by the tertiary structure, and is complementary to one substrate.
How can temperature and PH effect the enzyme?
It causes the enzyme to denature, because the bonds between the R-groups (disulfide, hydrogen and ionic bonds) are weakened or broken. This changes the active site, so the substrate is no longer complementary to the active site. Prevents the catalysing of the reaction.
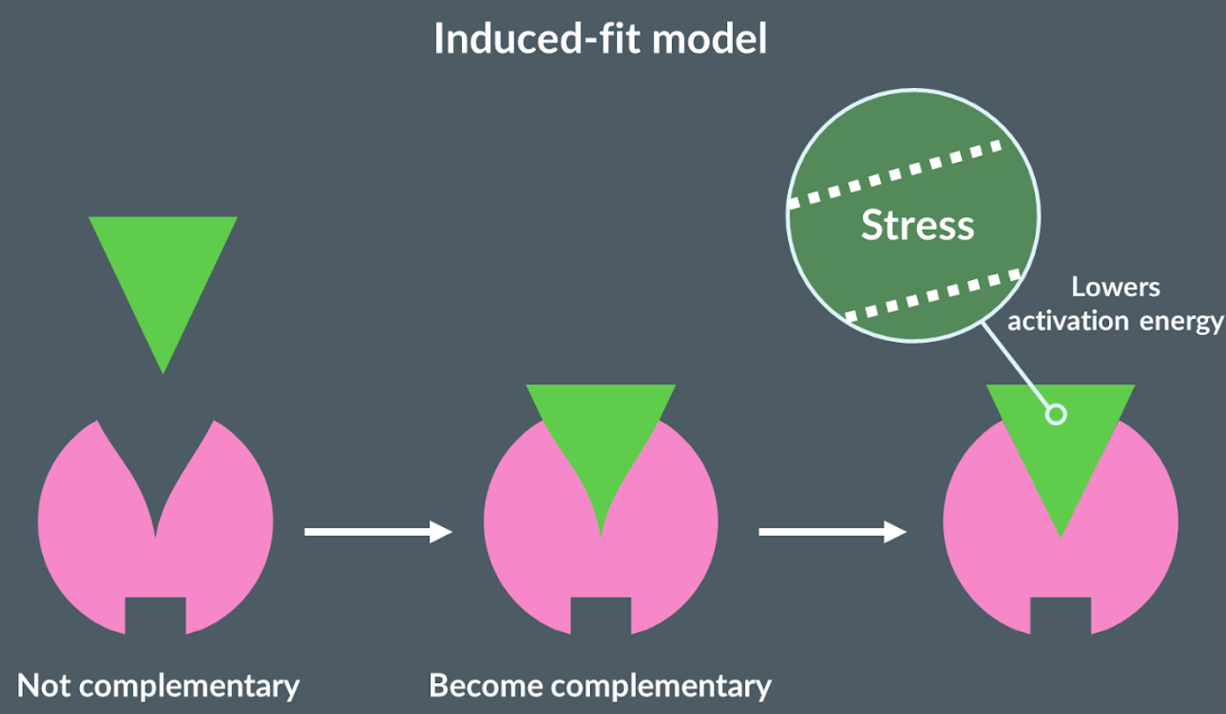
Induced-fit model
Proposes that initially the active site of the enzyme is not complementary to the substrate. Once the substrate binds to the active site, the substrate changes shape. This stresses the shape of the substrate which causes bonds to be broken easily, so lowers the activation energy.
six factors that effect the rate of reaction of enzyme
Concentration of substrate
Concentration of enzyme
Competitive inhibitors
Non-competitive inhibitors
Temperature
PH
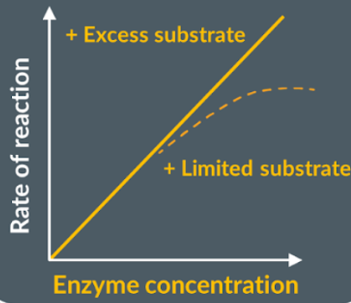
Enzyme concentration
Increasing the concentration of enzyme increases the amount of active sites available. So, there is a greater number of substrate-enzyme complexes which increases rate of reaction. Until it plateaus as not enough substrate, and not all active sites are used.
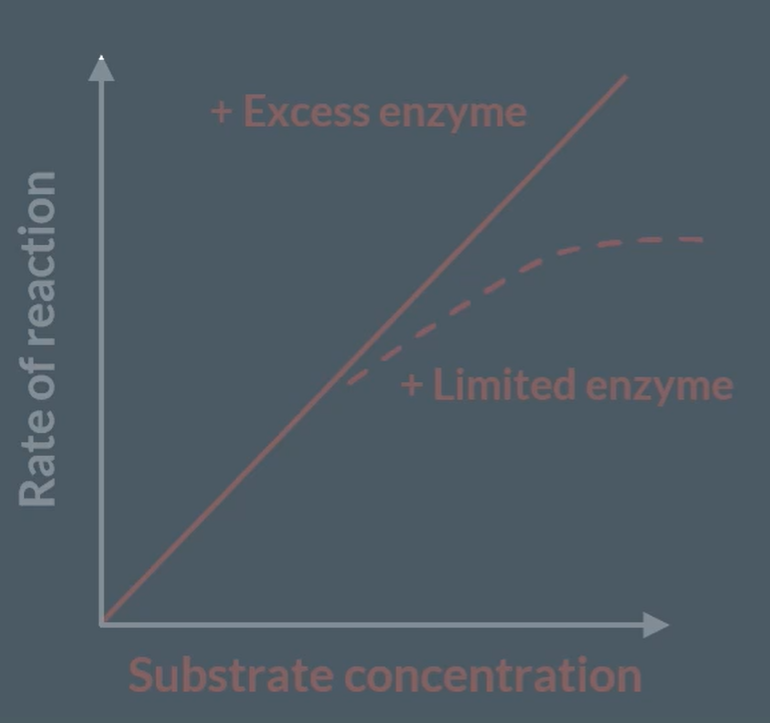
Substrate concentration
Increasing substrate concentration, increases rate of reaction as there is higher successful collisions, so increase number of enzyme-substrate complexes. However, the reaction plateaus as the limiting factor is the enzyme concentrations. Now, there are more concentration of substrates, but all active sites are being used up.
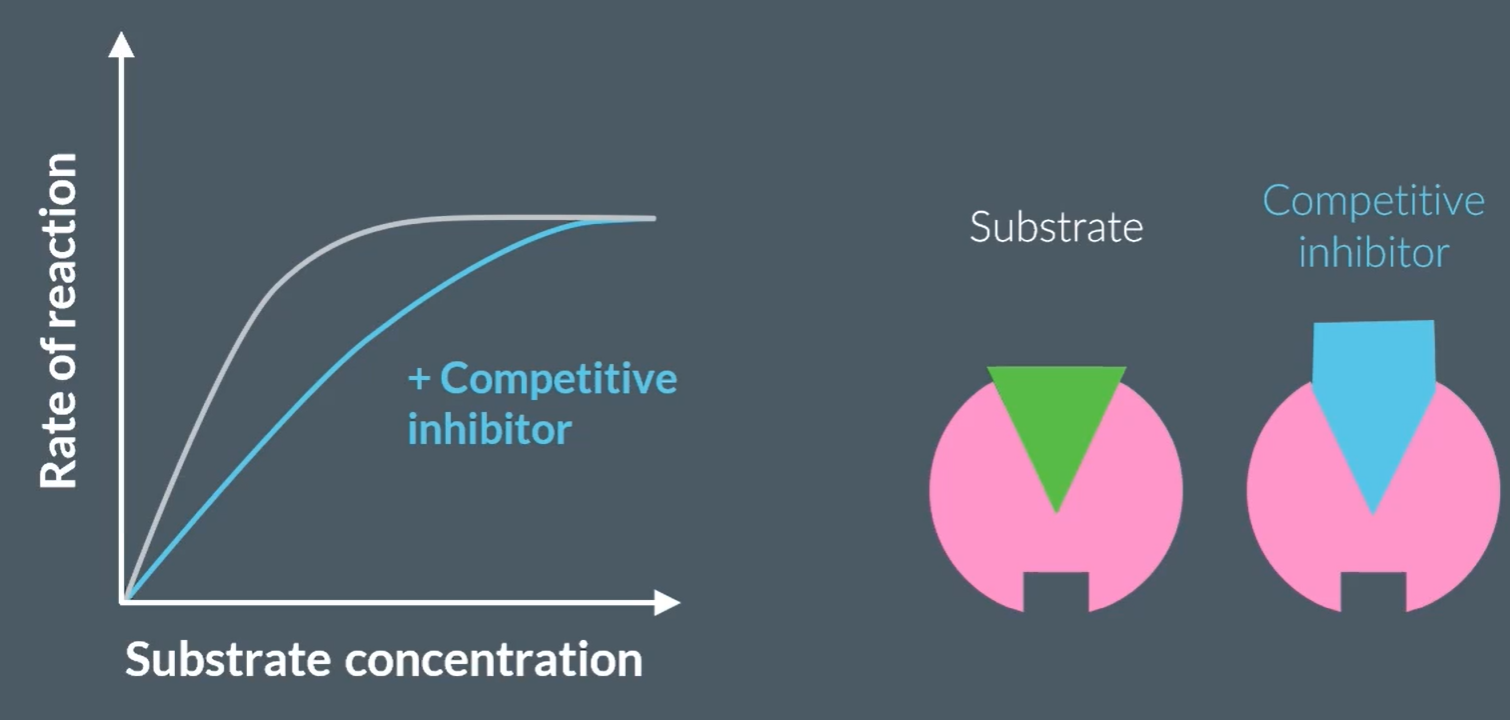
What is a competitive inhibitor?
They are another substrate that is complementary to the enzyme’s active site.
How does competitive inhibitor effect the rate of reaction?
They initially decrease the rate of reaction, because they are also complementary to the enzyme’s active site, so they temporarily occupy the enzyme. However, since this is temporary, the reaction between enzyme and substrate will complete, so plateaus afterwards.
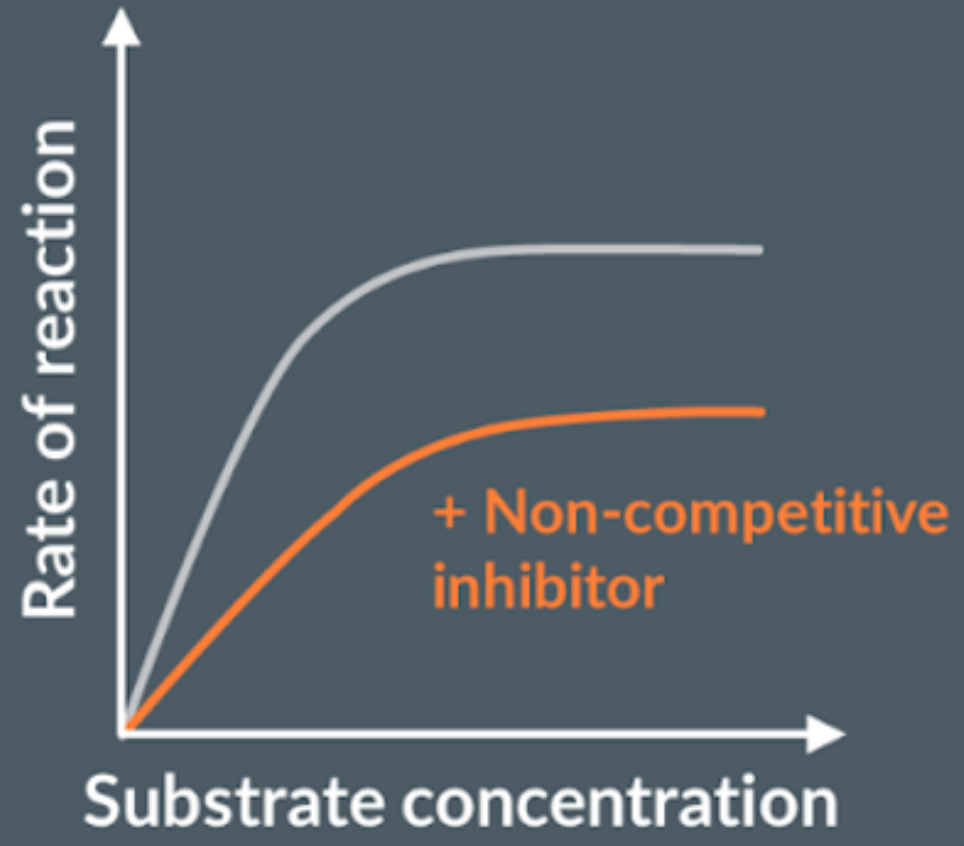
What are non-competitive inhibitors?
Don’t bind to enzymes active site, but another side of the enzyme, this alters the active site, preventing substrate to bind, and this is permanent.
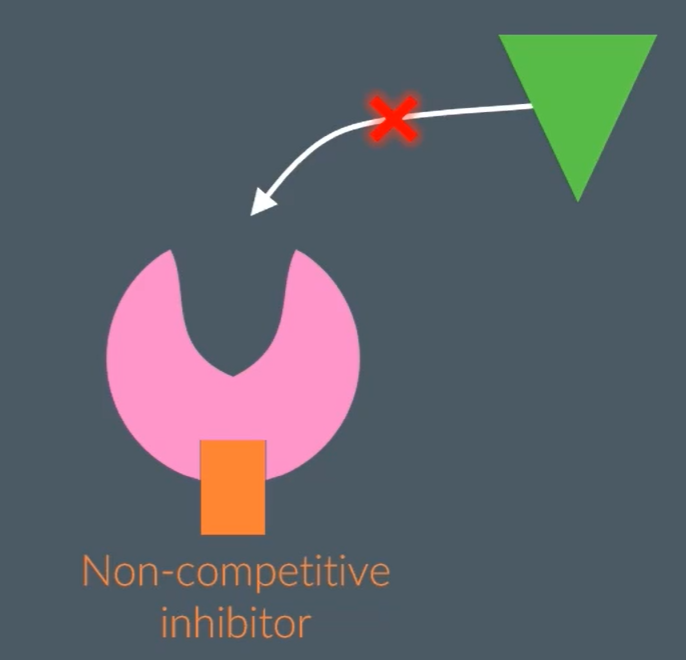
How do non-competitive inhibitors effect the rate of reaction?
The rate of reaction decreases, because the non-competitive inhibitor is not complementary to the enzyme’s active site but another part of the enzyme, which causes the active site of the enzyme to change shape, permanently. So, the rate of reaction plateaus regardless of substrate concentration increasing.
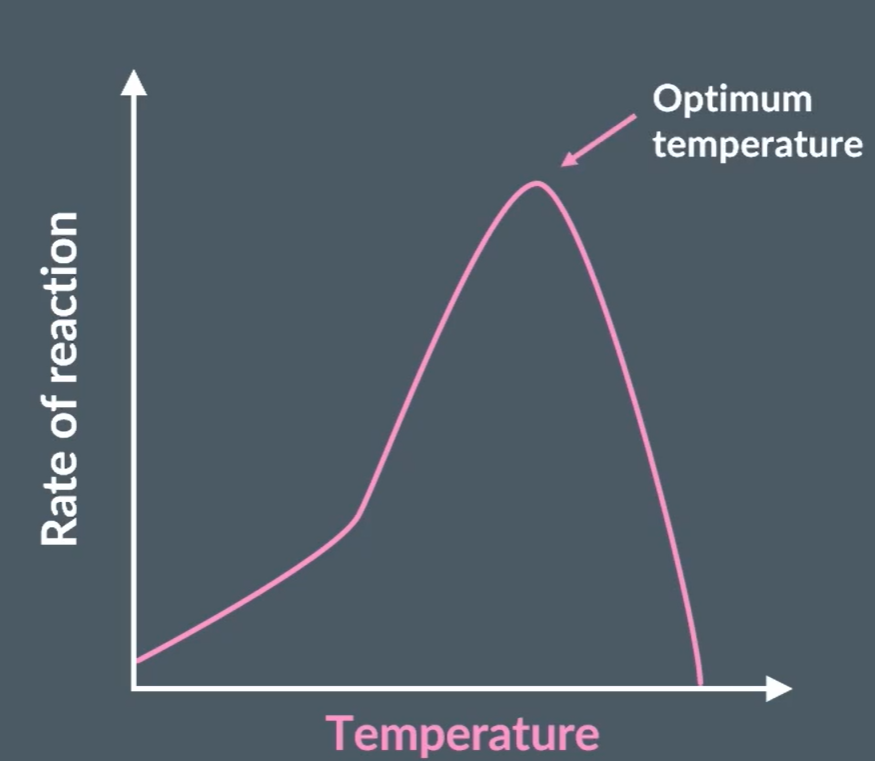
How does temperature effect the rate of reaction?
As the temperature increases the rate of reaction increases as there are more frequency of enzyme and substrate colliding in correct orientation, forming more enzyme-substrate complexes. After, optimum temperature the shape of the active site changes no longer binding to the substrate. This is because the bonds in the tertiary structure of the enzyme (disulfide, ionic and hydrogen bonds) are broken.
How does the PH of a solution effect the rate of reaction?
The PH of a solution is the measure of how many H+ ions there are. At large PH changes the H+ concentration alters the hydrogen and ionic bonds of the enzymes tertiary structure, therefore the active site of the enzyme. The PH alters the charge on the amino acid that form the active site of the enzyme.
PH Calculations
The Ph is determined by -log [concentration of H+ ions]
![<p>The Ph is determined by -log [concentration of H+ ions]</p>](https://knowt-user-attachments.s3.amazonaws.com/9b3a8ffa-c957-4b08-a8ad-606a350047ea.png)
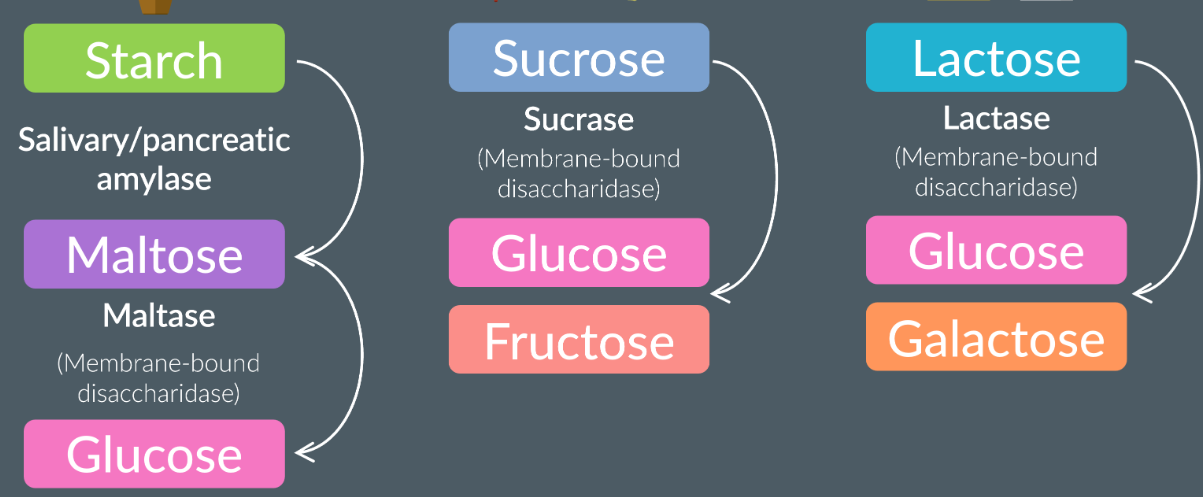
Digestion of carbohydrates: starch, sucrose, lactose
Happens in the mouth (salivary glands) and small intestine
The digestion of starch: the salivary glands release amylase which hydrolyses the glycosidic bonds and forms maltose. Any remaining starch molecules pass into the stomach and the small intestine (pancreas amylase). In the ileum, the maltase is a membrane-bound disaccardiase (attached to the epithelial membrane). Maltase hydrolyses the maltase to two alpha glucoses.
The sucrase (membrane-bound disacardiase) hydrolyses the sucrose to fructose and glucose in the ileum.
Lactase (membrane-bound disacardiase) hydrolyses lactose to glucose and galactose in the ileum.
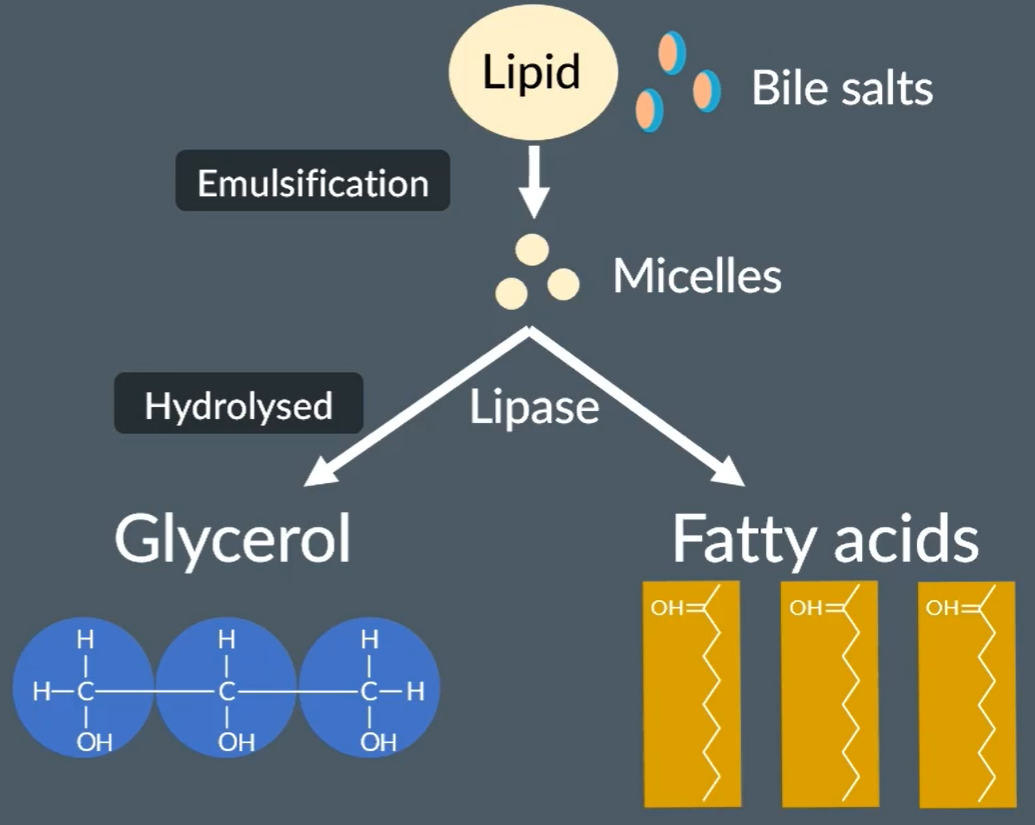
Digestion of lipids
Takes place in the small intestine
Lipids mix with bile salts, which undergo emulsification to form micelles.
Cholesterol is then added.
The micelles are then hydrolysed by lipase to form glycerol and fatty acids.
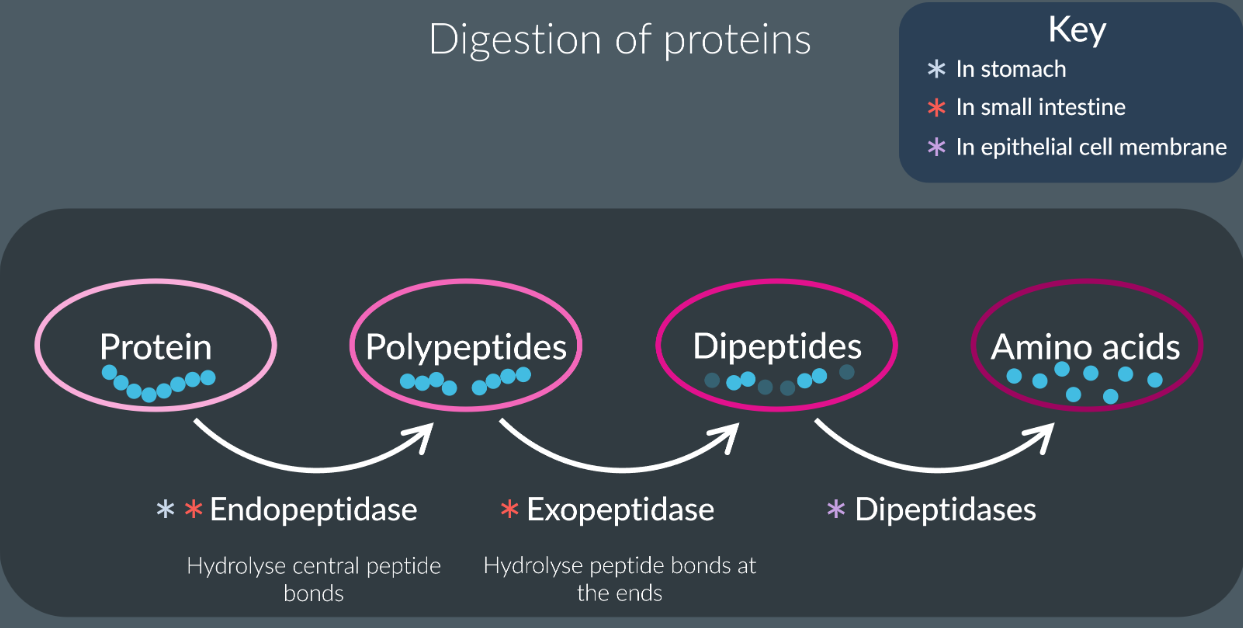
Digestion of proteins
They found in stomach and small intestine.
Endopeptidase hydrolyse the peptide bonds in the central region to form polypeptides.
Exopeptidases hydrolyse the peptide bonds at the end to form dipeptides and amino acids.
Dipeptidases are membrane bound found in epithelial cells hydrolyse the peptide bonds between the dipeptides to form amino acids.
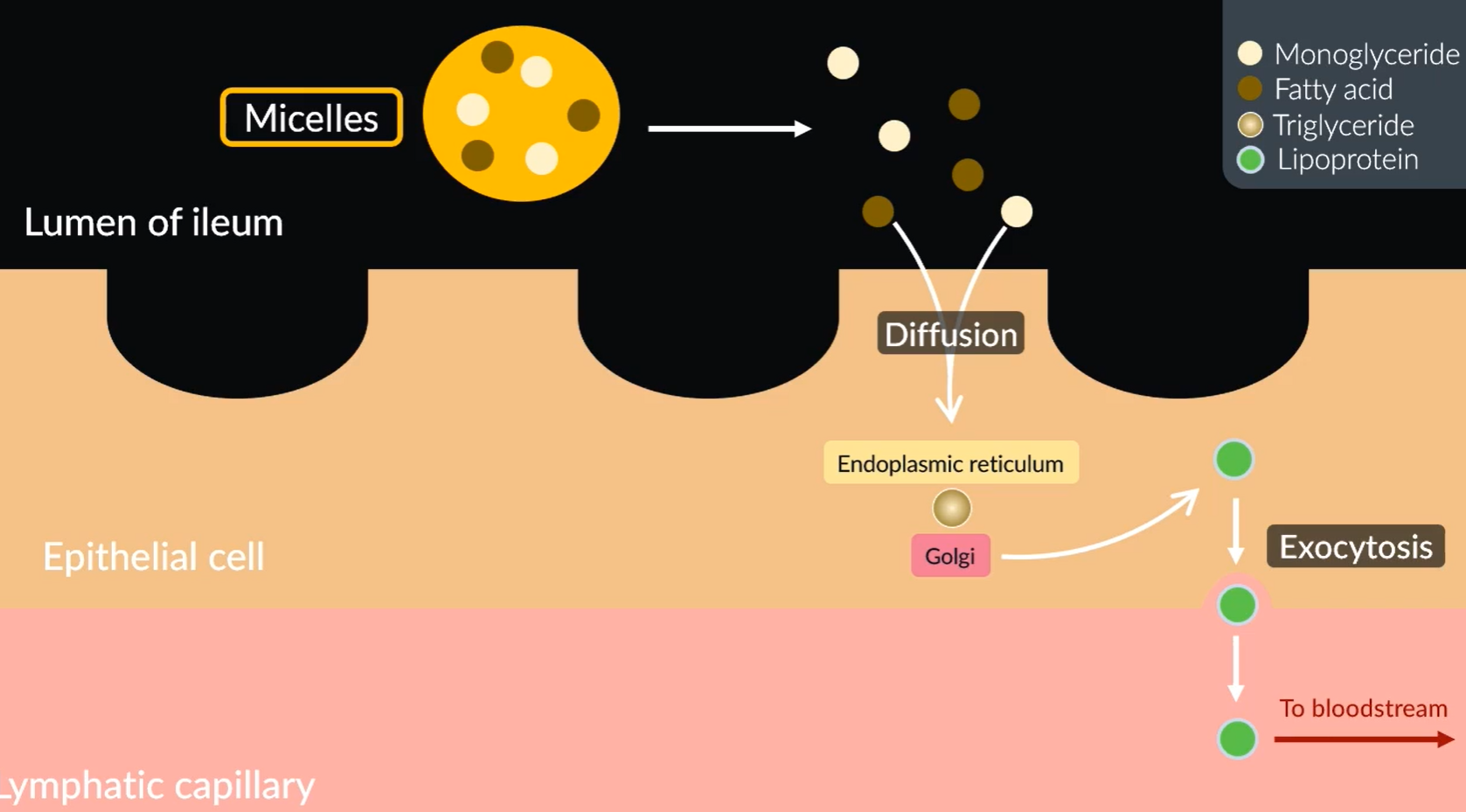
Absorption of lipids
Micelles move to the surface of the cell, where they break down to monoglycerides and fatty acids.
Since these are non-polar they diffuse into the epithelial cells.
Once inside, the endoplasmic reticulum turns them into triglycerides.
These then travel to the Golgi which get turned to lipoproteints (Chlyomicrons).
The lipoproteins (chylomicrons) move out of the epithelial cells into the lymphatic capillaries via exocytosis.
Which then travel into the blood stream. Once in the blood stream they get hydrolysed back into triglycerides.