Organic Chem 1 - Ch. 1-5
1/115
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
116 Terms
Isotopes
atoms with the same number of protons but different number of neutrons
atoms of the same element but with different atomic masses
Orbitals
an allowed energy state for an electron bound to a nucleus
the probability function that defines the distribution of electron density in space
up to two electrons can occupy each orbital if their spins are paired
Electron density
the relative probability of finding an electron in a certain region of space
Mass number
the sum of the protons and neutrons in an atom
Atomic number
the number of protons in an atom
Valence electrons
electrons on the outermost shell of the atom
Aufbau principle
states we must fill the lowest-energy orbitals first until we have the proper number of electrons
Hund’s Rule
states that when there are two or more orbitals of the same energy (degenerate), electrons will go into different orbitals rather than pairing up in the same orbital
Octet rule
atoms generally form bonding arrangements that give them filled shells of electrons (noble-gas configurations)
for the second-row elements, this configuration has eight valence electrons
elements in the third and higher rows can have an “expanded octet” of more than eight electrons
Ionic bond
bonding that occurs by the attraction of oppositely charged ions
usually results in the formation of a large, three-dimensional crystal lattice rather than individual molecules
common in inorganic compounds but relatively uncommon in organic compounds
Covalent bond
occurs by the sharing of electrons in the region between two nuclei
an unequally shared pair of bonding electrons is called a polar covalent bond
an equally shared pair of bonding electrons is called a nonpolar covalent bond
Lewis structure
a structural formula that shows all valence electrons, with the bonds symbolized by dashes or by pairs of dots, and nonbonding electrons symbolized by dots
allows you to see where all the electrons are and to determine if each atom obeys the octet rule
a correct/true Lewis structure should show any lone pairs
Nonbonding electrons
also called lone pairs
valence-shell electrons that are not shared between two atoms
lone pairs are nucleophiles — they participate in a host of chemical reactions
Valence
the number of bonds an atom usually forms
Dipole moment
a measure of the polarity of a bond/molecule, proportional to the product of the charge separation times the bond length
The electrons that contribute to an atom’s charge are…
all its unshared (nonbonding) electrons; plus
half the (bonding) electrons it shares with other atoms, or one electron of each bonding pair
Formal charge formula
formal charge (FC) = [group number] - [nonbonding electrons] - ½ [shared electrons]
Resonance hybrid
a molecule or ion for which two or more valid Lewis structures can be drawn, differing ONLY in the placement of the valence electrons
Resonance structures: general rules
All the resonance forms must be valid Lewis structures for the compound. Second-row elements (B, C, N, O, F) can never have more than eight electrons in their valence shells.
Only the placement of the electrons many be shifted from one structure to another. Electrons in double bonds and nonbonding electrons (lone pairs) are most commonly shifted.
Nuclei cannot be moved, and all bond angles must remain the same.
Sigma bonds are very stable, and they are rarely involved in resonance.
The major resonance contributor is the one with the lowest energy. The best contributors generally have the most octets satisfied, as many bonds as possible, and as little charge separation as possible.
Electronegative atoms such as N, O, and halogens often help to delocalize positive charges, but they can bear a positive charge only if they have octets.
Resonance stabilization is most important when it serves to delocalize a charge or a radical over two or more atoms.
Condensed structural formula
written without showing all of the individual bonds— each central atom is shown together with the atoms that are bonded to it; if there are two or more identical groups, parentheses and a subscript may be used to represent all the identical groups
Line-angle formula
a shorthand structural formula with bonds represented by lines— carbon atoms are implied wherever two lines meet or a line begins or bends; hydrogen atoms are not shown unless they are on a drawn atom
Molecular formula
the number of atoms of each element in one molecule of a compound
Empirical formula
the ratio of atoms in a compound
Molecular orbital
an orbital formed by the overlap of atomic orbitals on different atoms
Hybrid atomic orbital
a directional orbital formed from a combination of s and p orbitals on the same atom
Valence-shell electron-pair repulsion (VSEPR) theory
bonds and lone pairs around a central atom tend to be separated by the largest possible angles: about 180 degrees for two, 120 degrees for three, and 109.5 degrees for four
sp hybrid orbitals
two orbitals (s and p) combine to form two sp orbitals
linear electron pair geometry
180-degree bond angle
sp2 hybrid orbitals
three orbitals (one s and two p) combine to form three sp2 orbitals
trigonal planar geometry
120-degree bond angle
sp3 hybrid orbitals
four orbitals (one s and three p) combine to form four sp3 orbitals
the atom has tetrahedral electron-pair geometry
109.5-degree bond angle
Constitutional (structural) isomers
isomers that differ in the order in which their atoms are bonded together
Stereoisomers (configurational isomers)
isomers whose atoms are bonded together in the same order but differ in how atoms are oriented in space — example: cis- vs. trans-
Cis-trans (geometric) isomers
stereoisomers that differ in their cis-trans arrangement on a double bond or on a ring; must have two different groups on each end of the double bond
the cis isomer has similar groups on the same side
the trans isomer has similar groups on opposite sides
Functional group
the reactive, non-alkane part of an organic molecule
a specific combination of atoms that has a unique set of chemical properties
Dipole-dipole forces
attractive intermolecular forces resulting from the attraction of the positive and negative ends of the permanent dipole moments of polar molecules
van der Waals forces
the attractive forces between neutral molecules, including dipole-dipole forces and London dispersion forces
London dispersion forces
intermolecular forces resulting from the attraction of correlated temporary dipole moments induced in adjacent molecules
Hydrogen bond
a particularly strong attraction between a nonbonding pair of electrons and an electrophilic O—H or N—H hydrogen
Three main factors to consider when predicting relative boiling points:
hydrogen bonding
molecular weight and surface area
dipole moments
Hydrophobic
means “water-hating” — nonpolar substances or groups that do not readily dissolve in water (water-repellant)
Hydrophilic
means “water-loving” — polar substances or groups that readily dissolve in water (water-attractive)
Arrhenius acids/bases
acid: substance that dissociates in water to give hydronium ions
base: substance that dissociates in water to give hydroxide ions
Bronsted-Lowry acids/bases
acid: any species that can donate a proton
base: any species that can accept a proton
Lewis acids/bases
bases: species with available electrons that can be donated to form new bonds — also called nucleophiles
acids: species that can accept these electron pairs to form new bonds — also called electrophiles
Curved-arrow formalism
a method of drawing curved arrows to keep track of electron movement from nucleophile to electrophile (or within a molecule) during the course of a reaction
Hydrocarbons
Compounds composed exclusively of carbon and hydrogen.
major classes: alkanes, alkenes, alkynes, aromatic hydrocarbons
nearly all are nonpolar or only slightly polar
tend to be hydrophobic

Alkanes
Hydrocarbons containing only single bonds.
generally have the -ane suffix, and the first part of the name indicates the number of carbon atoms in the chain
Cycloalkanes
a special class of alkanes in the form of a ring
Alkyl group
an alkane portion of a molecule, with one hydrogen atom removed to allow bonding to the rest of the molecule; can be represented with the symbol R
Alkenes
Hydrocarbons that contain carbon-carbon double bonds.
the double bond is the most reactive part, so it is considered the functional group
end in the -ene suffix
these bonds cannot rotate — show geometric (cis-trans) isomerism
Alkynes
Hydrocarbons with carbon-carbon triple bonds as their functional group.
generally have the -yne suffix
triple bond is linear, so there is no cis-trans isomerism
to accommodate a triple bond, four atoms must be in a straight line —> cycloalkynes are rare and must contain at least eight carbon atoms
Aromatic hydrocarbons (arenes)
Hydrocarbons containing a benzene ring— a six-membered ring with three double bonds.
look like cycloalkenes, but have different properties
have a very stable bonding arrangement
when it serves as a substituent, it is called a phenol group
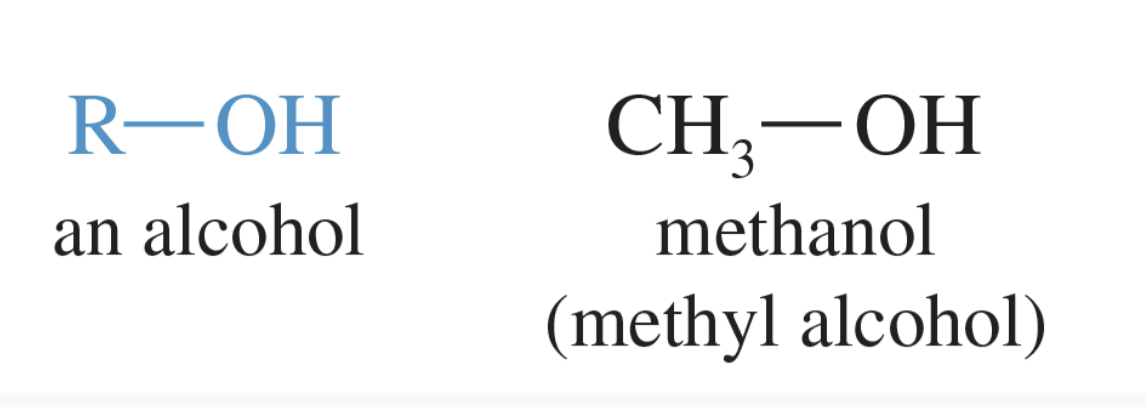
Alcohols
Organic compounds that contain the hydroxy group (—OH) as their functional group.
the general formula is R—OH
classified as primary, secondary, or tertiary depending on whether the hydroxy group is bonded to a primary, secondary, or tertiary carbon atom
among the most polar organic compounds —> hydrophilic
end in the -ol suffix
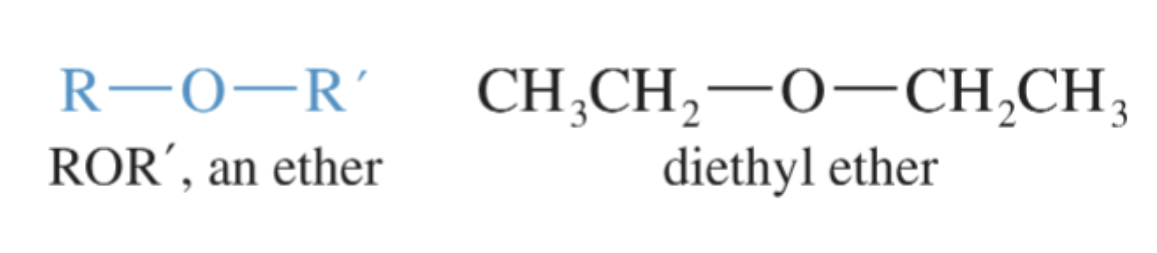
Ethers
Compounds with two alkyl groups bonded to an oxygen atom.
the general formula is R—O—R’ (the R’ means another alkyl group, which may or may not be the same as the first R)
much more polar than hydrocarbons
since they have no O—H hydrogens, they cannot hydrogen bond with themselves
names are often formed from the names of the alkyl groups + the word “ether”
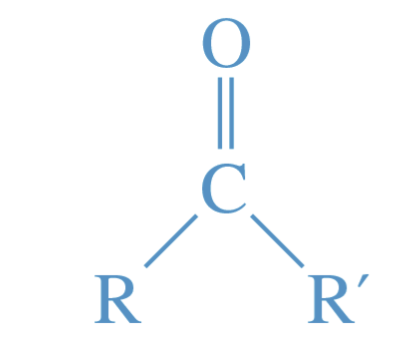
Carbonyl group
The C=O functional group.
contains both aldehydes and ketones
strongly polar—> hydrophilic
can form hydrogen bonds with hydrogen-bond doners such as water, alcohols, and amines
Ketone
A compound containing a carbonyl group bonded to two alkyl groups.
names generally have the -one suffix
contains a carbonyl functional group (C=O)
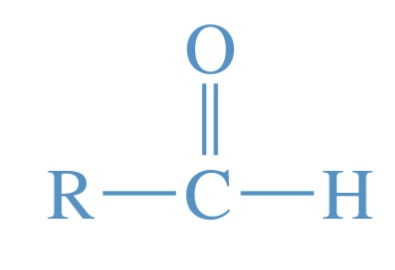
Aldehyde
A compound containing a carbonyl group bonded to an alkyl group and a hydrogen atom.
names generally use the -al suffix or -aldehyde suffix
contains a carbonyl functional group (C=O)
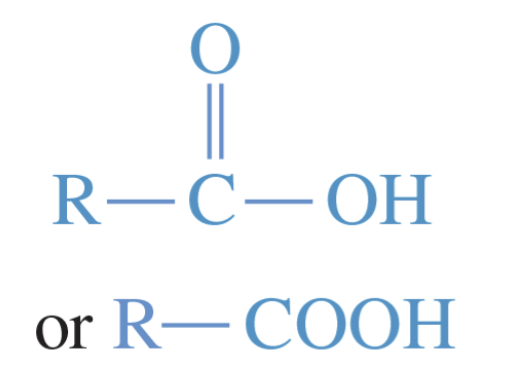
Carboxylic acids
Any compounds containing the carboxyl group, —COOH as their functional group.
general formula is R—COOH
owe their acidity (pKa is about 5) to the resonance-stabilized carboxylate anions formed by deprotonation
often uses historical names; systemic names would use -oic acid suffix
strongly polar —> hydrophilic
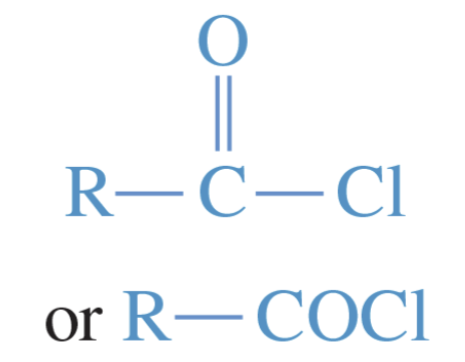
Acid chloride
an acid derivative with a chlorine atom in place of the hydroxyl group

Ester
an acid derivative in which the hydroxy group of the acid is replaced by an alkoxy group
it is a composite of a carboxylic acid + an alcohol
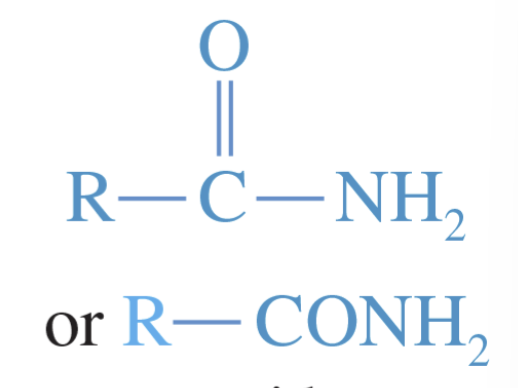
Amide
an acid derivative in which the hydroxy group of the acid is replaced by a nitrogen atom and its attached hydrogens or alkyl groups
it is a composite of a carboxylic acid + an amine or ammonia
among some of the most stable acid derivatives; form very weak bases
form particularly strong hydrogen bonds —> strongly polarized
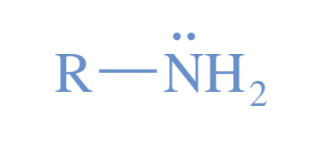
Amine
A derivative of ammonia with one or more alkyl groups bonded to the nitrogen atom.
because of their basicity, naturally-occurring amines are often called alkaloids
simple amines are named by naming the alkyl groups bonded to nitrogen and adding the word “amine”
nitrogen atom is hydrophilic
Nitrile
A compound containing the cyano group.
cyano group = a functional group that includes a carbon-nitrogen triple bond
strongly polar; most small nitriles are soluble in water
sp-hybridized bonding group
Common names
the names that have developed historically, generally with a specific name for each compound; also called trivial names
IUPAC (systematic) names
the systematic names that follow the rules adopted by the International Union of Pure and Applied Chemistry
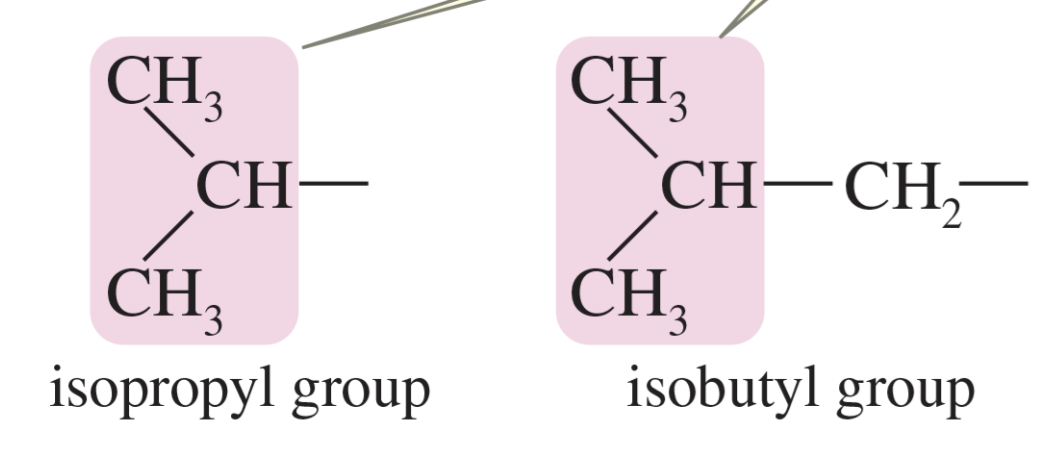
“iso” grouping
branched alkyl group that has the formula (CH3)2CH
Degree of alkyl substitution
The number of alkyl groups bonded to a carbon atom in a compound or in an alkyl group.
n- means primary (bonded to one other carbon atom)
sec- means secondary (bonded to two other carbon atoms)
tert- means tertiary (bonded to three other carbon atoms)
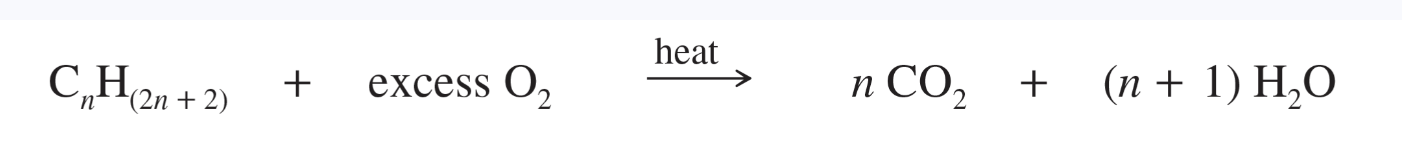
Combustion reaction
a rapid oxidation at high temperatures in the presence of air or oxygen
Catalytic cracking
heating large alkanes to cleave them into smaller molecules
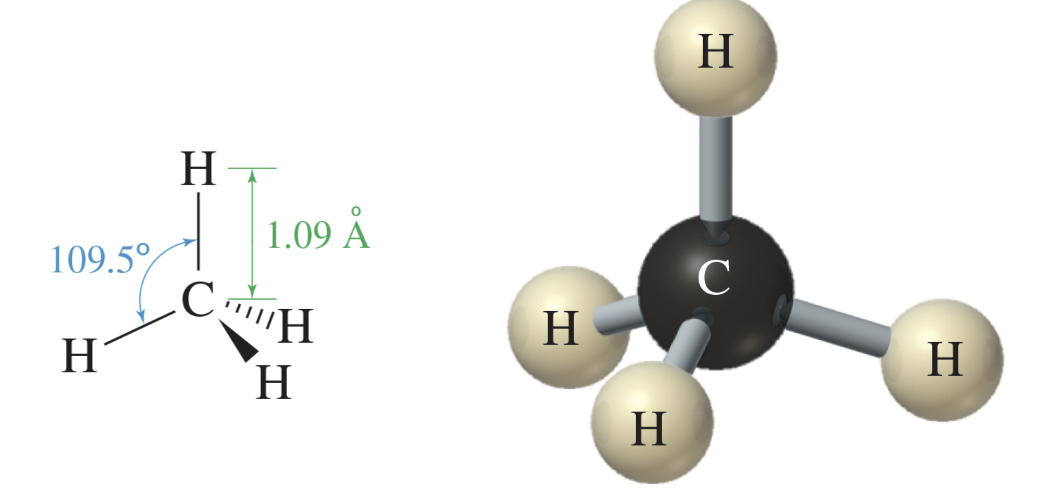
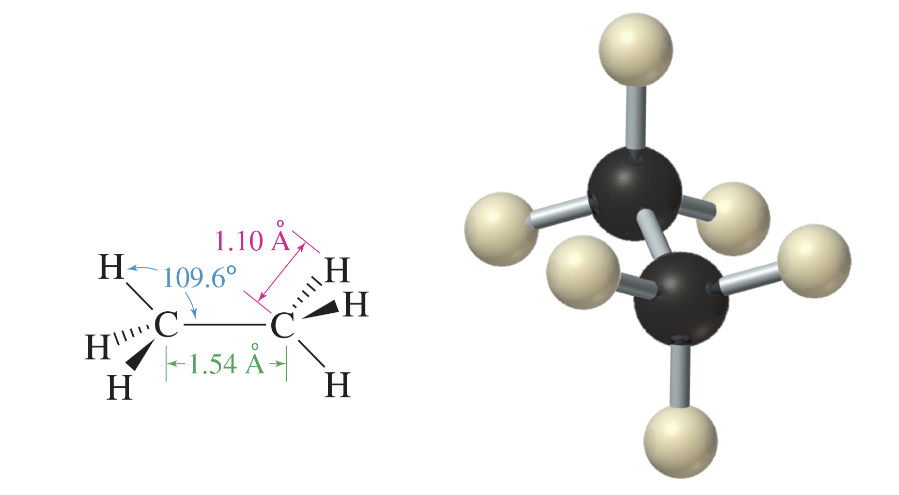
Methane; CH4
simplest alkane
perfectly tetrahedral
109.5 degree bond angles predicted for an sp3 hybridized carbon
four hydrogen atoms are covalently bonded to the central carbon atom
Ethane
two-carbon alkane
composed of two methyl groups with overlapping sp3 hybrid orbitals forming a sigma bond between them
methyl groups are not fixed in a single position, and are able to rotate about the sigma bond that connects the two
infinite number of conformations possible
Conformations
different arrangements of atoms caused by rotation about a single bond
Newman projections
A way of drawing the conformations of a molecule by looking straight down the bond connecting two carbon atoms
front carbon atom is represented by three lines (bonds) coming together in a Y shape
the back carbon is represented by a circle with three bonds pointing out from it
torsional strain
the strain associated with eclipsing of bonds in the ring
torsional energy
the energy required to twist a bond into a specific conformation
guache conformation
a conformation with a 60-degree dihedral angle between the largest groups
anti conformation
a conformation with a 180-degree dihedral angle between the largest groups; usually the lowest-energy conformation
steric strain
the interference between two bulky groups that are so close together that their electron clouds experience a repulsion
angle strain
sometimes called Baeyer strain; the strain associated with distorting bond angles to smaller (or larger) angles
ring strain (in cyclic compounds)
the extra strain associated with the cyclic structure of a compound, as compared with a similar acyclic compound
composed of angle strain and torsional strain
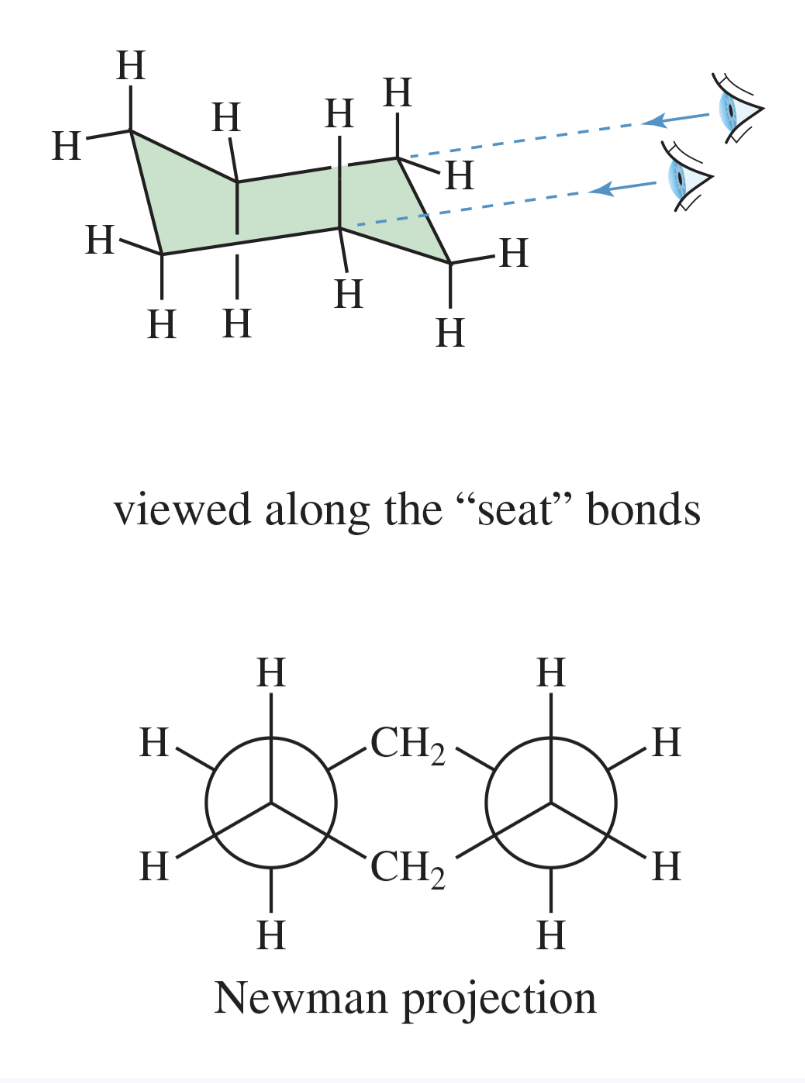
chair conformation
the most stable conformation of cyclohexane, with one part puckered upward and one part puckered downward
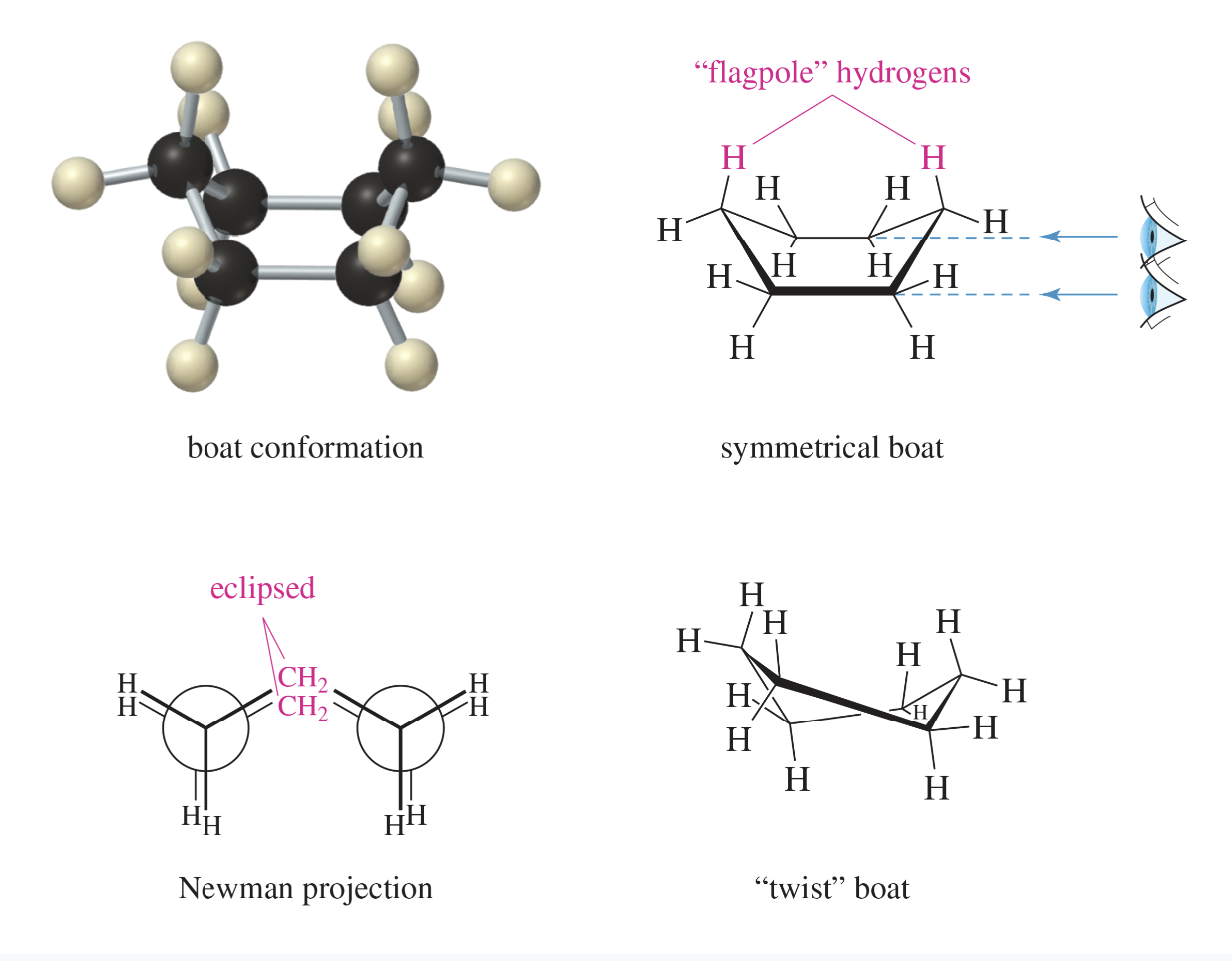
boat conformation
the less stable puckered conformation of cyclohexane, with both parts puckered upward
the most stable boat is actually the twist boat conformation; twisting minimizes torsional and steric strain
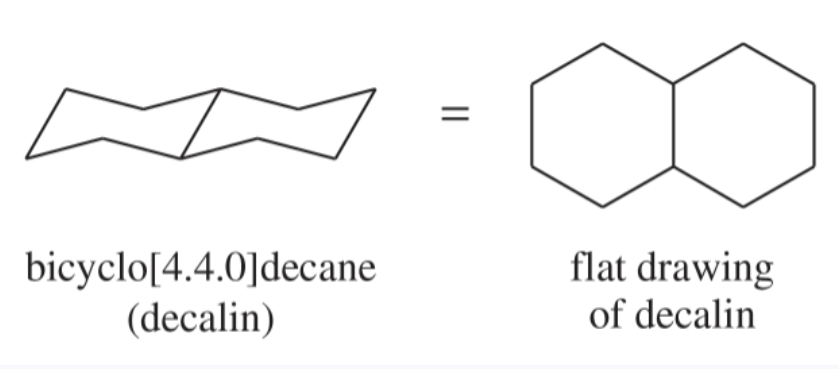
fused rings
rings that share a common carbon-carbon bond and its two carbon atoms
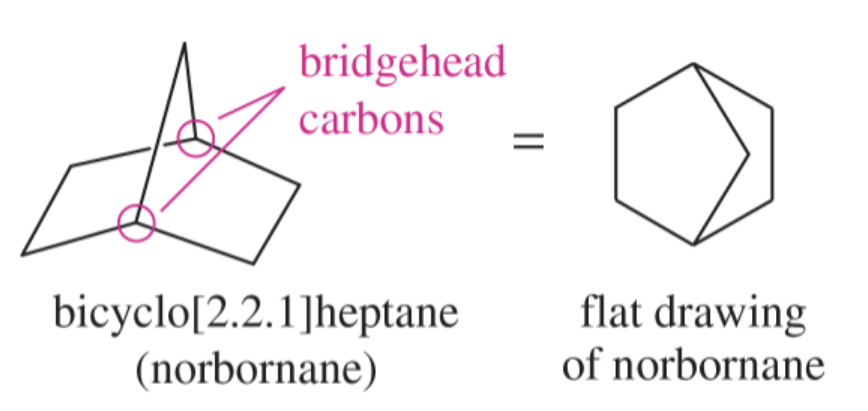
bridgehead carbons
the carbon atoms shared by two or more rings in a bridged bicyclic compound
three chains of carbon atoms (bridges) connect the bridgeheads
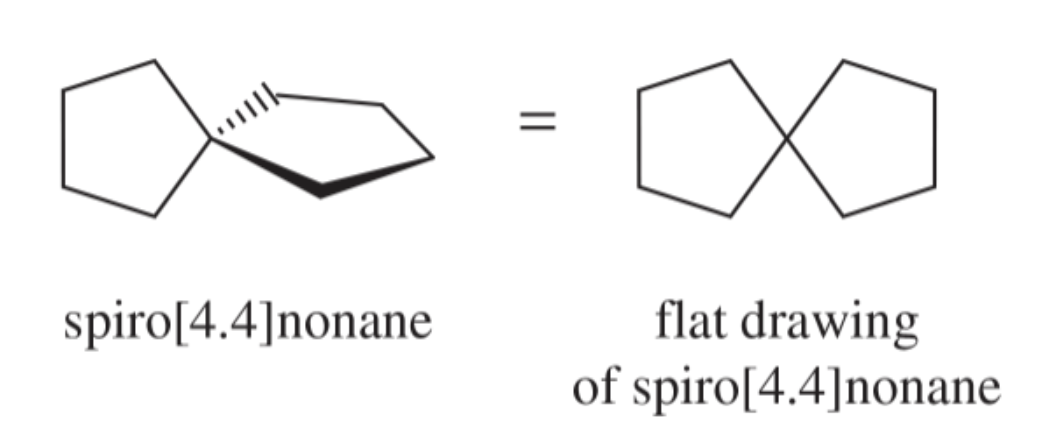
spirocyclic compounds
bicyclic compounds in which the two rings share only one carbon atom
mechanism
the complete, step-by-step description of exactly which bonds break and which bonds form, and in what order to give the observed products
Thermodynamics
the study of the energy changes that accompany chemical and physical transformations
allows us to compare the stability of reactants and products and predict which compounds are favored by the equilibrium
Kinetics
the study of reaction rates, determining which products are formed fastest
also helps to predict how the rate will change if the reaction conditions are altered
chain-reaction mechanism
a multistep reaction where a reactive intermediate formed in one step brings about a second step that generates the intermediate needed for the following step
initiation step
the preliminary step in a chain reaction, where the reactive intermediate is first formed
propagation steps
the steps in a chain reaction that are repeated over and over to form the product
the sum of the propagation steps should give the net reaction
termination steps
any steps where a reactive intermediate is consumed without another one being generated
substitution (displacement) reaction
a reaction in which an attacking species (nucleophile, electrophile, or free radical) replaces another group
reactive intermediate
a short-lived species that is never present in high concentration because it reacts as quickly as it is formed
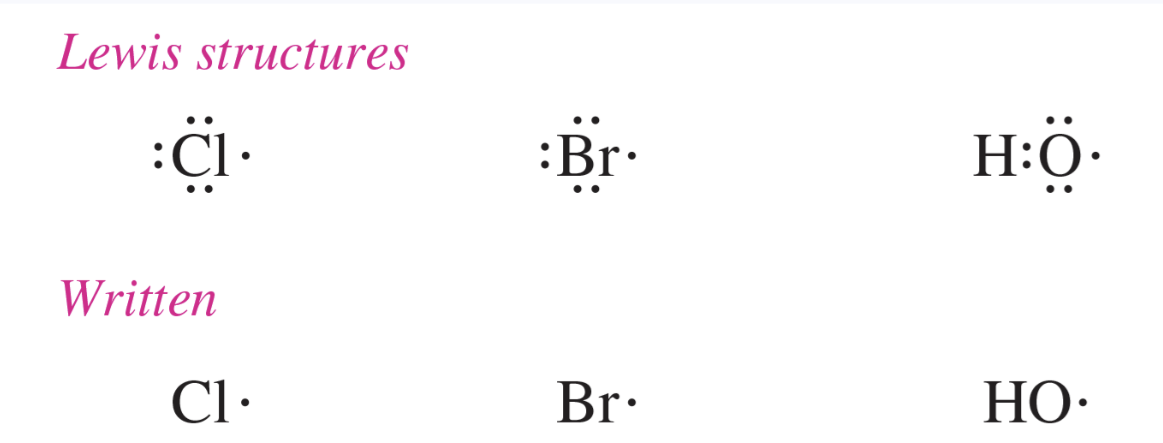
free radical
a highly reactive species in which one of the atoms has an odd number of electrons
most commonly, a radical contains a carbon atom with three bonds and an “odd” (unpaired) electron
Four main ways that electrons move in polar reactions:
Nucleophilic attack on electrophile
Loss of a Leaving Group — heterolytic bond cleavage, an atom or group takes the electron pair
Proton Transfers (Acid/Base)
Rearrangements — carbocations can be stabilized by neighboring groups through slight orbital overlapping called hyperconjugation
Equilibrium
a state of a system such that no more net charge is taking place
the rate of the forward reaction equals the rate of the reverse reaction
Equilibrium constant
a quantity calculated from the relative amounts of the products and reactants present at equilibrium
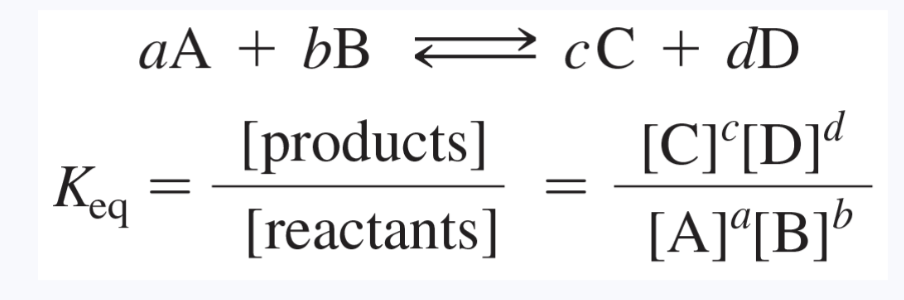
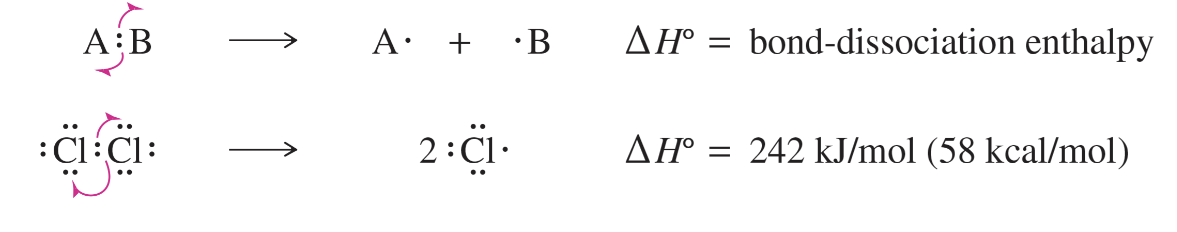
homolytic (radical) cleavage
the breaking of a bond in such a way that each atom retains one of the bond’s two electrons —> produces two free radicals
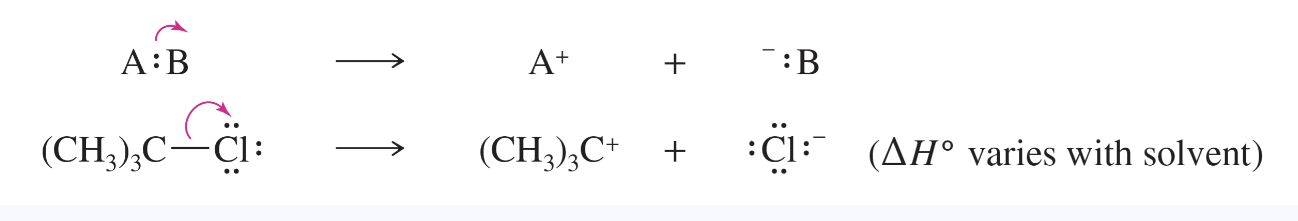
heterolytic (ionic) cleavage
the breaking of a bond in such a way that one of the atoms retains both of the bond’s electrons —> forms two ions
transition state (activated complex)
the state of highest energy between reactants and products
a relative maximum (high point) on the reaction-energy diagram
unlike the reactants or products, it is unstable and cannot be isolated