Membranes, Diffusion, Osmosis
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
functions of membranes
1) Regulate the passage of substances into and out of cells and between cell organelles and cytosol
*regulated by proteins on the membrane; membranes separates ICF and ISF
2) Detect chemical messengers arriving at the cell surface
*hormones, NT (usually outside the cell and bind to receptors on cell surface to cause reaction inside side)
3) Link adjacent cells together by membrane junctions
4) Anchor cells to the extracellular matrix
*membrane proteins
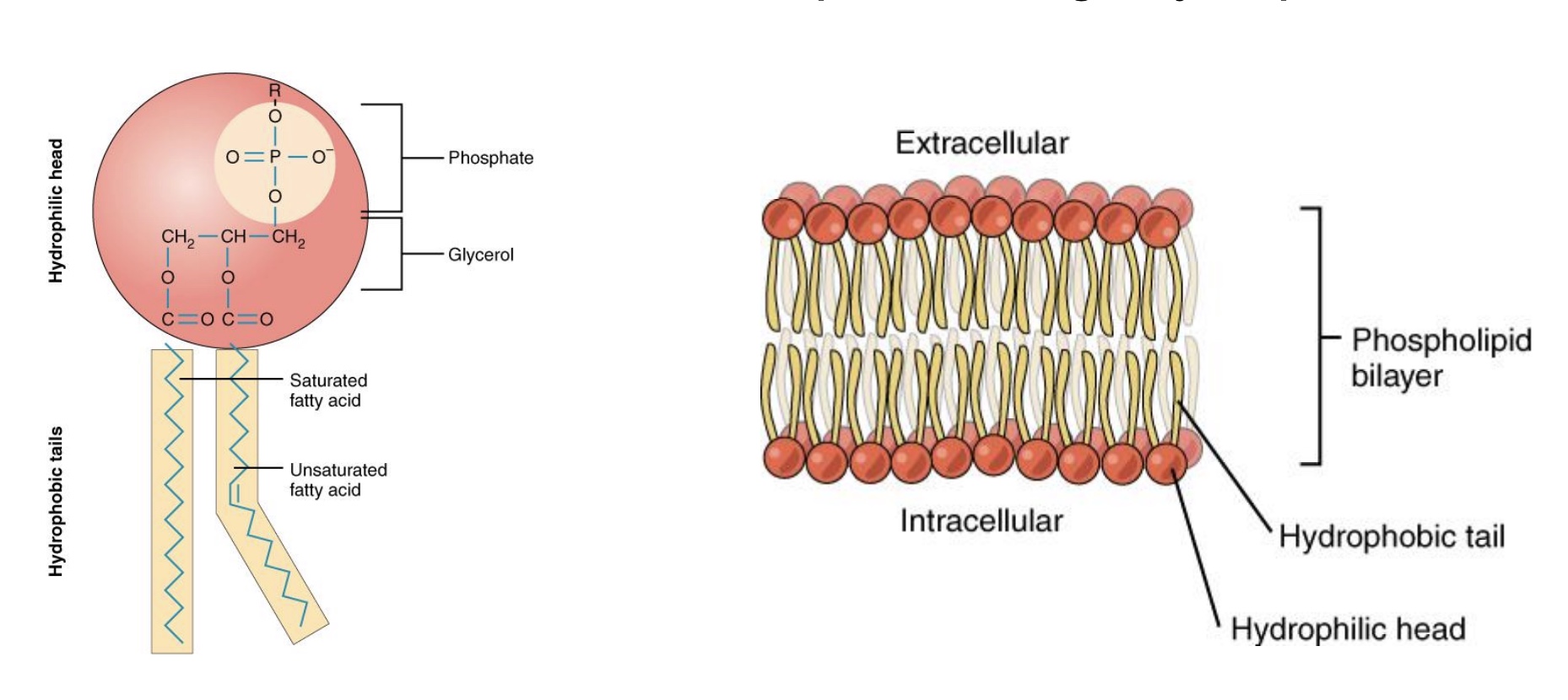
plasma membrane structure
-Phospholipid bilayer
Hydrophilic heads: toward watery environment/ISF/cytosol, both sides
*water soluble
Hydrophobic fatty-acid tails: inside membrane
-Ions and water-soluble molecules cannot pass through hydrophobic/non-polar membrane interior
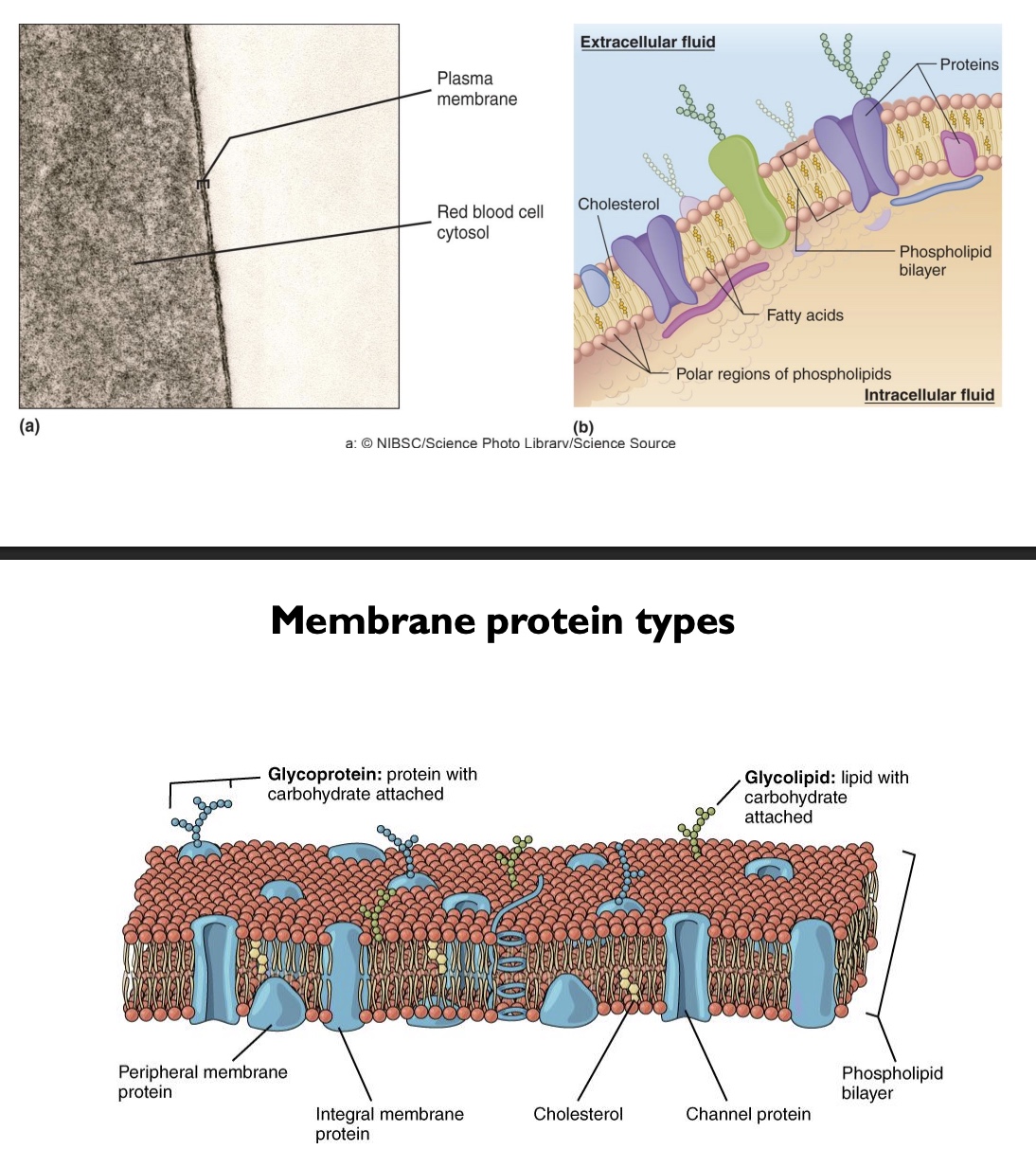
membrane structures and protein types
-Cholesterol: so more fluid and flexible, scattered throughout
-Channel proteins
-Integral membrane proteins: embedded
-Peripheral membrane proteins: don’t go all the way across
-Glycoproteins: proteins with CHO chains attached (so immune system can identify)
-Glycolipids: phospholipids with a CHO attached
-Plasma membrane itself relatively thin
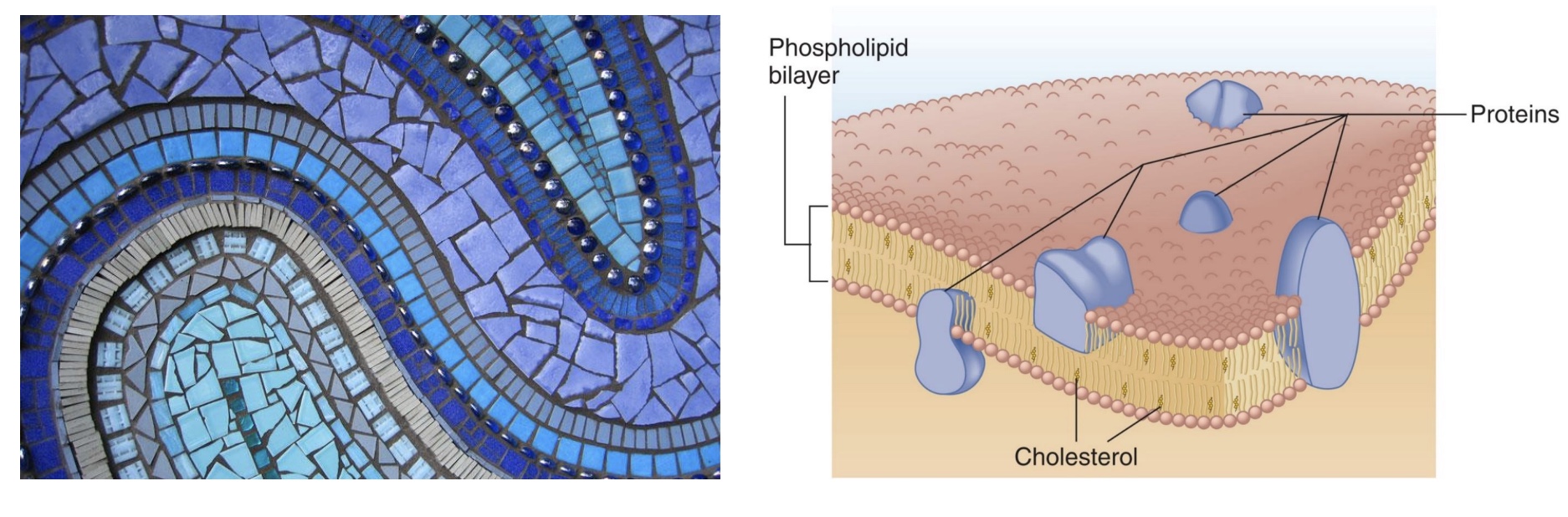
fluid mosaic model
to describe the plasma membrane bc made of lots of little pieces the make up the whole thing, and is flexible
Membrane junctions (list + simple definition)
linking cells
-Desmosomes: strong attachment between cells or cells and connective tissue
-Tight junctions: form a barrier (so water, etc. can’t pass through)
-Gap junctions: allow ions and some other molecules to pass for communication
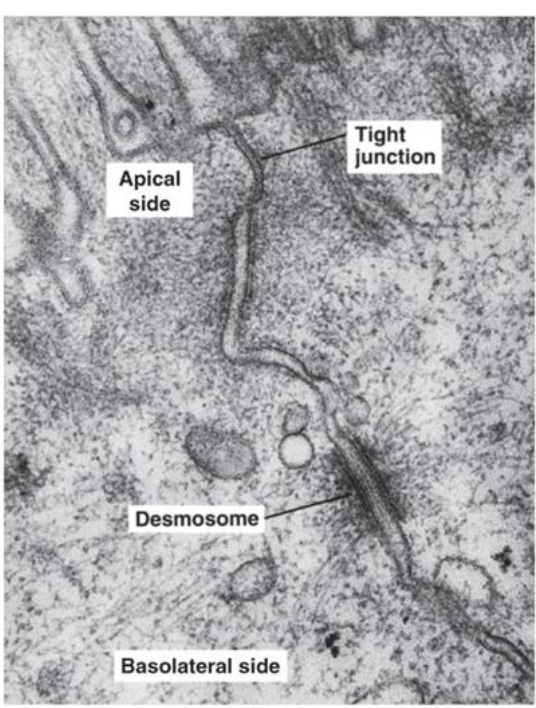
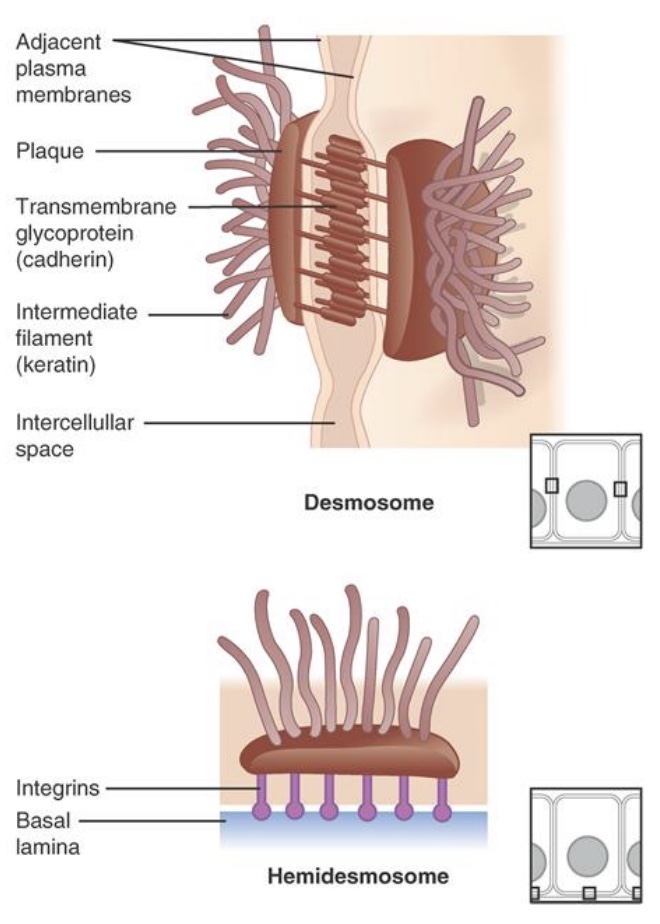
desmosomes
-provide strong attachments between cells
-cytoskeleton: web of proteins that helps hold the shape
-proteins interlock so strong
-ex: skin
-Hemidesmosome: at the junction of connective tissue and the basement membrane; under epithelial cells
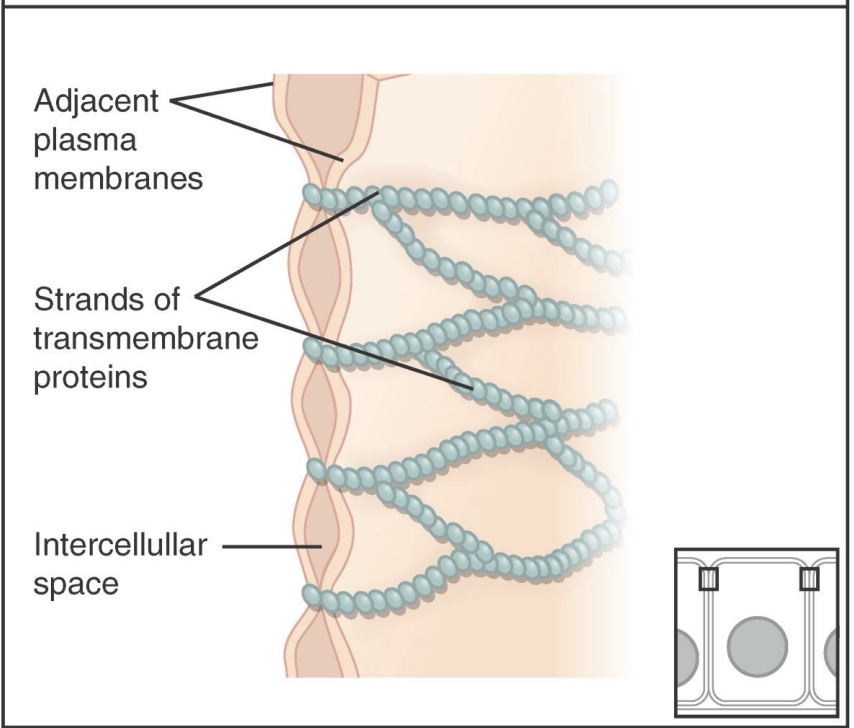
Tight Junctions
-prevent most substances from leaking between cells; makes the epithelial layer less leaky
-on the apical side/outside
-ex: kidney nephron
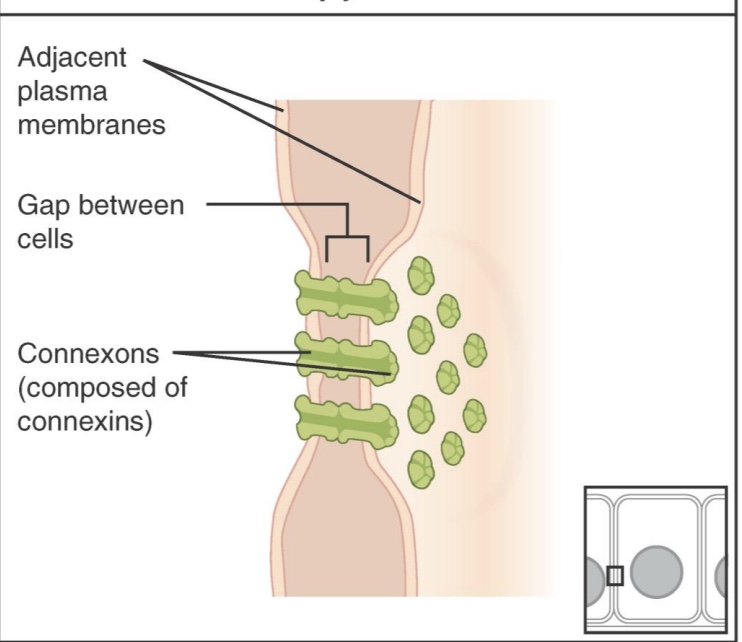
Gap Junctions
-allow for communication between neighboring cells
-Connexons: two hemi-channels
-ex: heart muscle (AP can pass cell to cell)
Brownian Motion
-random molecular motion that drives diffusion

Diffusion
-Movement of particles down a concentration gradient, from area of high concentration toward area of low concentration
-Driven by random molecular motion (aka Brownian Motion)
-Over time, reaches equilibrium:
• particles evenly distributed
• particles still moving, but no net movement (Brownian motion still there)
*ex: BG, O2 and other gases
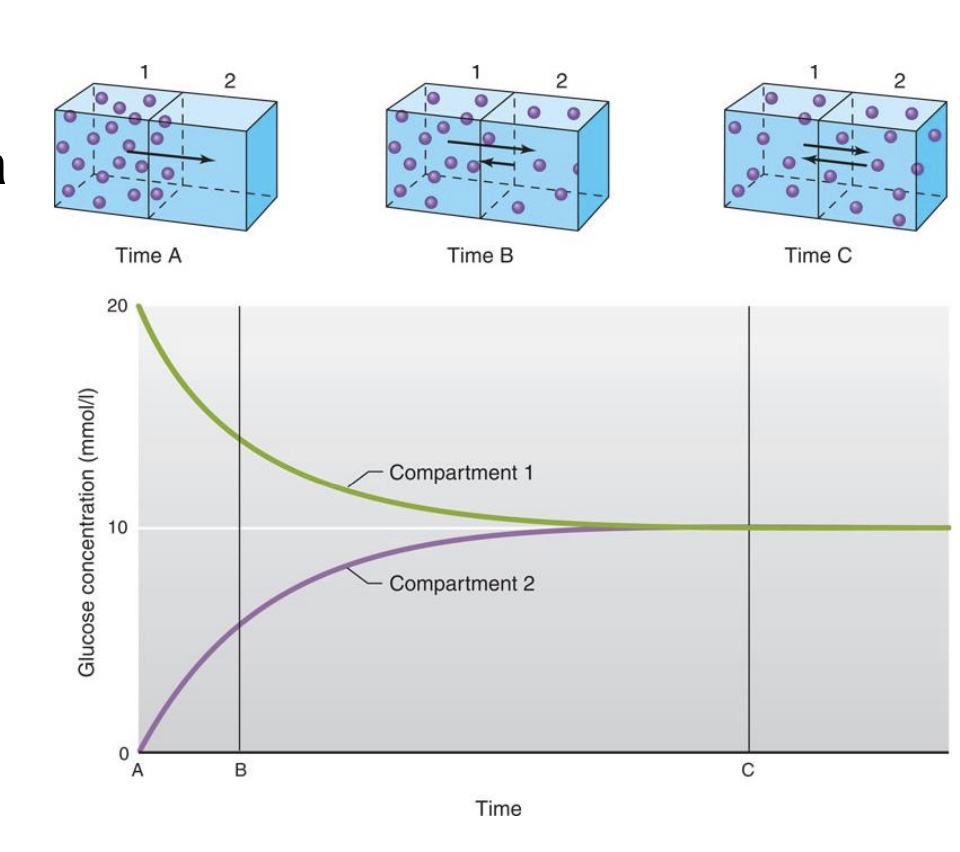
“net flux”
-In this example, membrane is freely permeable to particles; Random motion causes crossing of membrane (flux) in both directions; Net flux is from high to low
*”flux”= move into cell
-no net flux at equilibrium (last picture; but still moving and across the membrane)
Factors affecting diffusion
-Many factors can affect diffusion, but few of them change significantly during normal life processes
• Temperature: measure of molecular motion, but not too relevant to phys since pretty constant
• Medium/solvent: (water) not much change in phys
• Molecular weight: occasionally relevant (larger molecules diffuse slower)
• Surface area: increase SA= increase diffusion rate, relatively constant in phys (but theoretically could effect, ex: remove part GI villi, alveoli blocked could lead to pneumonia)
• Membrane permeability: from freely → non-permeable, channel proteins, good only under certain conditions
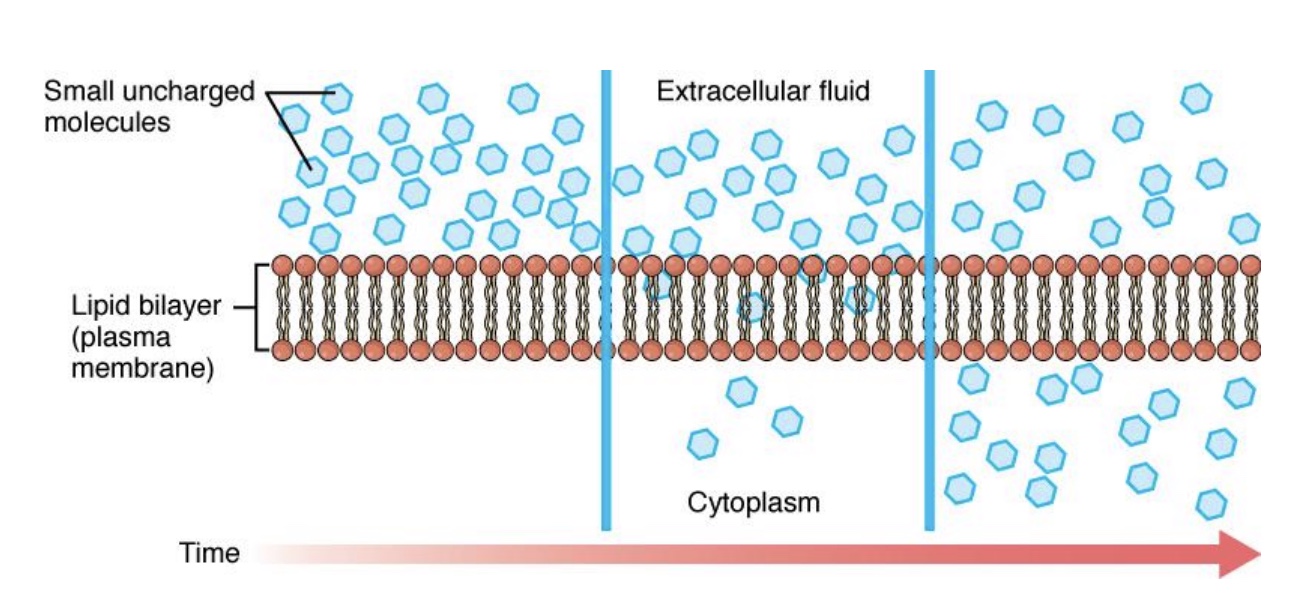
polar vs nonpolar diffusion through membranes
-Because the interior of the lipid bilayer is nonpolar, polar molecules cannot diffuse through
Includes ions, most drugs & nutrients, water
-Nonpolar molecules are able to diffuse (*don’t need a special carrier)
• Steroid hormones
• Oxygen
• Carbon dioxide
simple diffusion
-through the membrane though the lipid bilalyer (if nonpolar) OR through a channel (if small, polar molecules)
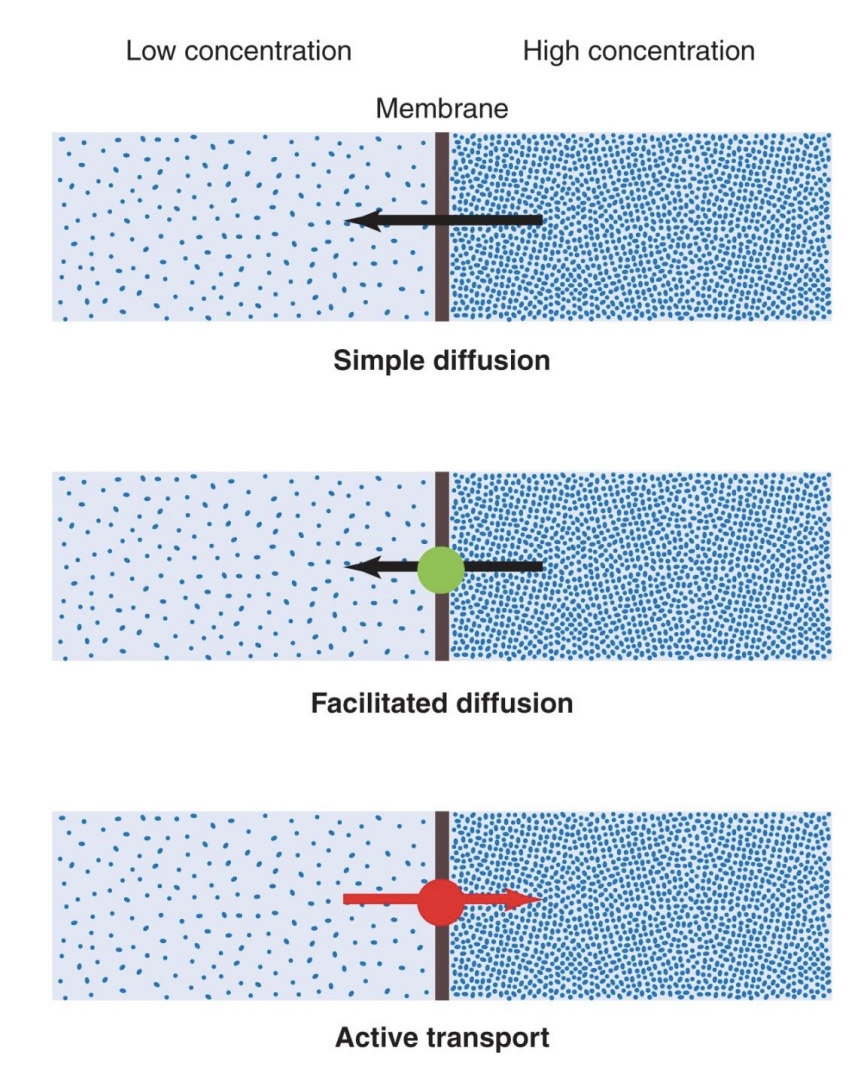
carrier-mediated transport
-Carrier-mediated transport: moves large molecules OR transports against the concentration gradient (low → high)
• Facilitated diffusion: substances move down concentration gradient (no energy required, similar to simple diffusion where flow is down concentration gradient, but needs a carrier protein)
• Active transport: substances move up concentration gradient (energy required)
*need protein that changes shape
•Primary: pumps that directly use ATP
•Secondary: energy comes from concentration gradient of a different solute
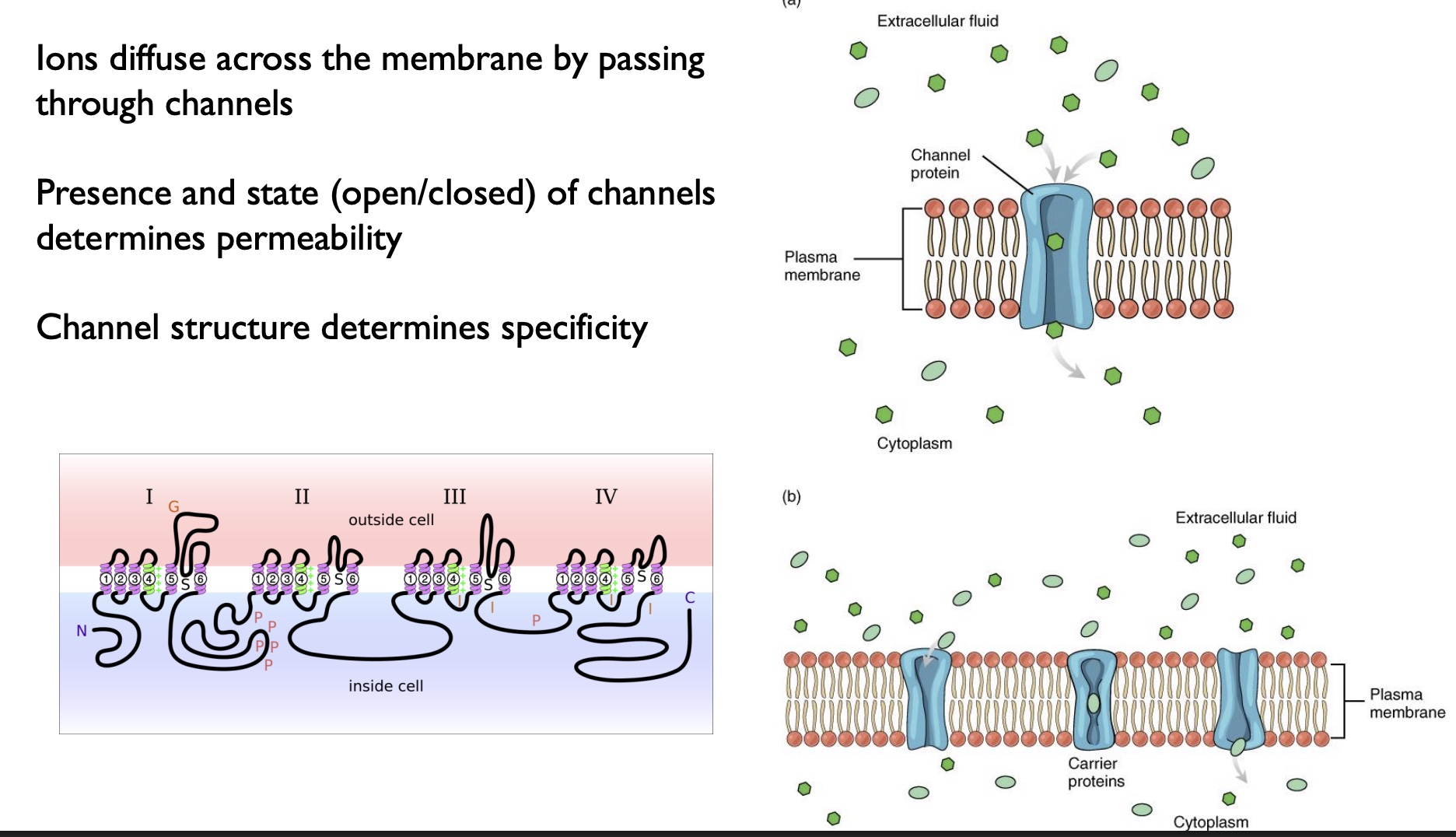
different types of channels and how used
-Ions diffuse across the membrane by passing through channels
-Presence and state (open/closed) of channels determines permeability (*based on stimuli)
-Channel structure determines specificity
*picture… both=no energy needed
Simple Diffusion (top)
-can move rapidly, speed determined by the concentration gradient
-can go direct through membrane OR through a channel protein
Facilitated Diffusion (bottom)
-molecules have to temporality bind to the carrier, then the carrier changes shape and transports the molecule to the other side
-rate-limited bc of the binding w/ carrier!!! (can become saturated)
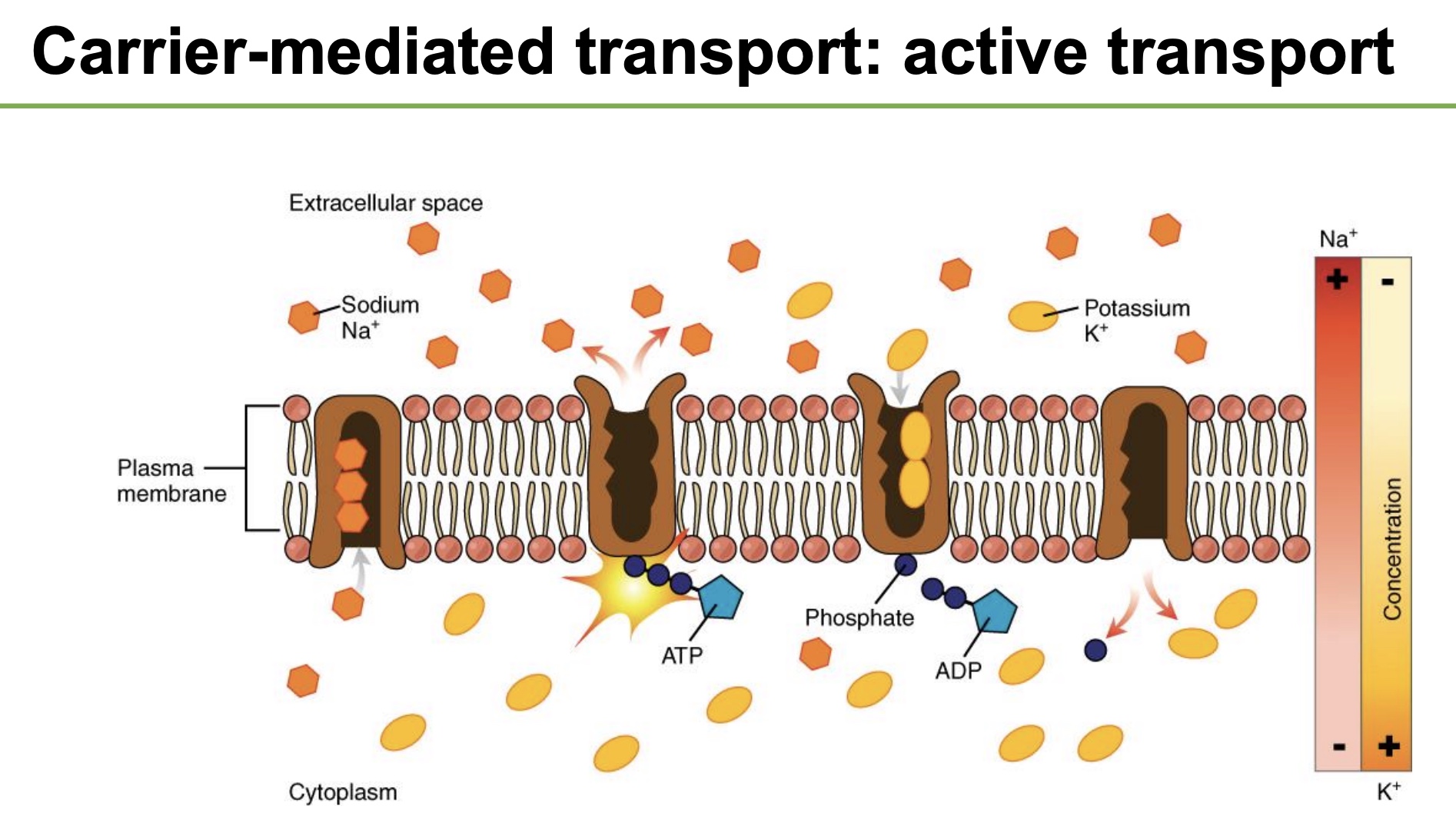
Na+K+ Pump
-moves both up/against their gradient, and thus is an ATPase and uses 1 ATP (form of “primary” active transprot)
-3Na+ out, 2K+ in, 1 ATP used
-important in all cells, on almost all membranes, helps establish gradients
Diffusion vs Carrier-Mediated Transport rate limits
-In simple diffusion, flux is limited only by the concentration gradient.
*higher [ ] gradient= higher flux
-In carrier-mediated transport, the number of available carriers places an upper limit on the rate of flux. Mediated transport can be saturated
*levels off/no additional flux/physical limit of transportation
ex: DM- glucose in urine bc saturate transporters
![<p>-In <strong>simple diffusion</strong>, flux is limited only by the <strong>concentration gradient.</strong></p><p>*higher [ ] gradient= higher flux</p><p>-In <strong>carrier-mediated transport,</strong> the number of available carriers places an upper limit on the rate of flux. Mediated transport <strong>can be saturated</strong></p><p>*levels off/no additional flux/physical limit of transportation</p><ul><li><p>ex: DM- glucose in urine bc saturate transporters</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/25a9dab9-d682-4aaf-914e-03d125e3811c.jpg)
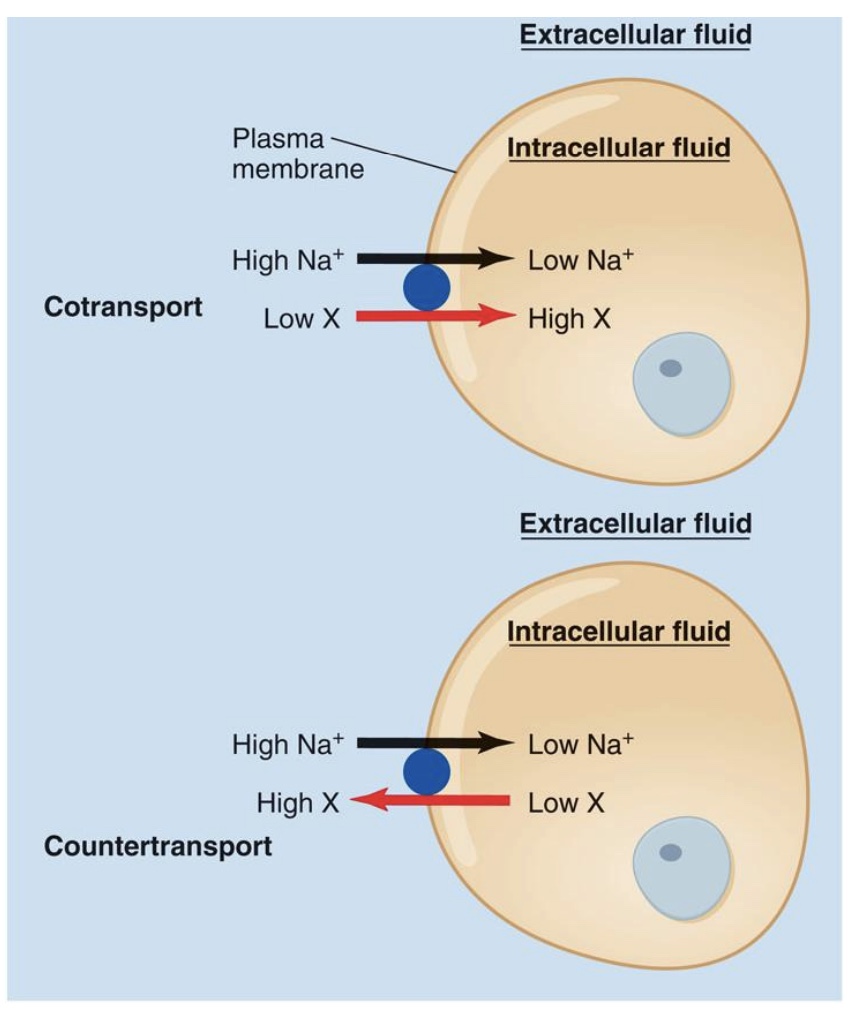
Forms of secondary active transport
-Secondary active transport: uses the energy of an existing gradient
*no direct use of ATP, but still use energy
Cotransport: the ion and the second solute cross the membrane in the same direction
ex: BG
Countertransport: the ion and the second solute move in opposite directions.
Why Secondary?
-Energy of Na+ gradient is used; This gradient was originally established by primary active transport/ Na+k+ pump
*both can capture energy from downhill energy of Na+
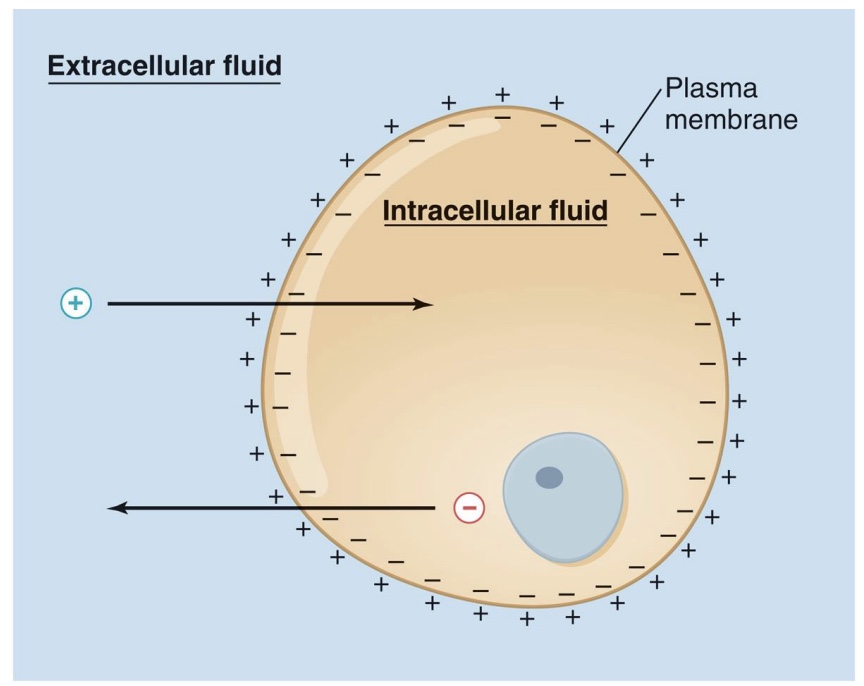
membrane potential and electrochemical gradient
-Because the membrane is selective, there are different ion concentrations inside and outside the cell
-This results in an electrical charge: membrane potential
Have a charge difference bc membrane is semi-permeable and bc of Na+K+ pump
-electrochemical gradient: sum of electrical and chemical (diffusion) gradients
-overall: negative inside cell, positive outside cell
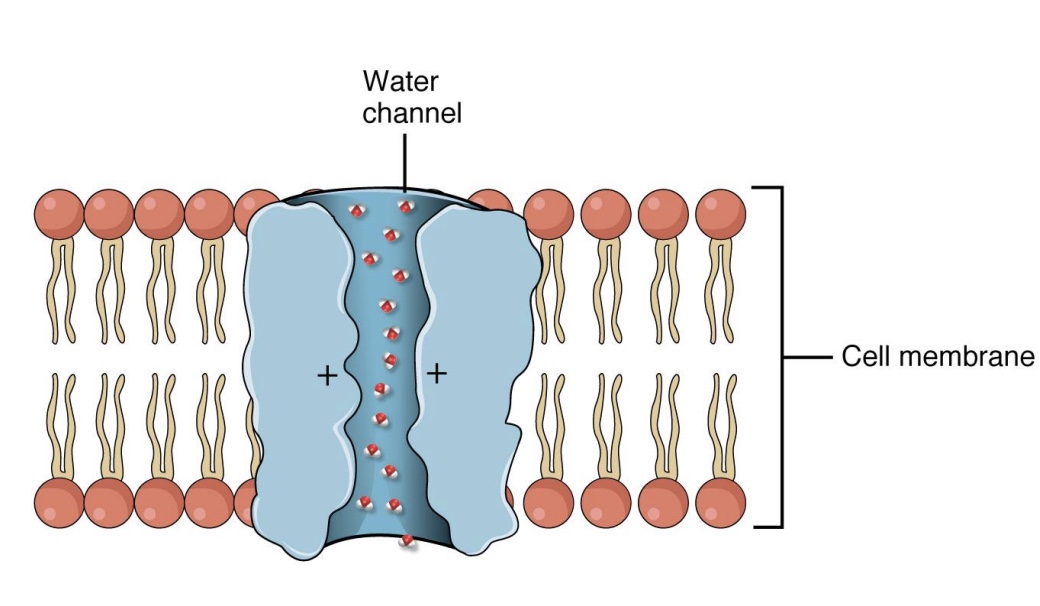
Osmosis
-Movement of water into and out of cells
-Special channels called aquaporins
-Like any other substance, water moves down concentration gradient (high water conc → low water conc)
*special case of diffusion; some water can go across, but usually needs an aquaporin
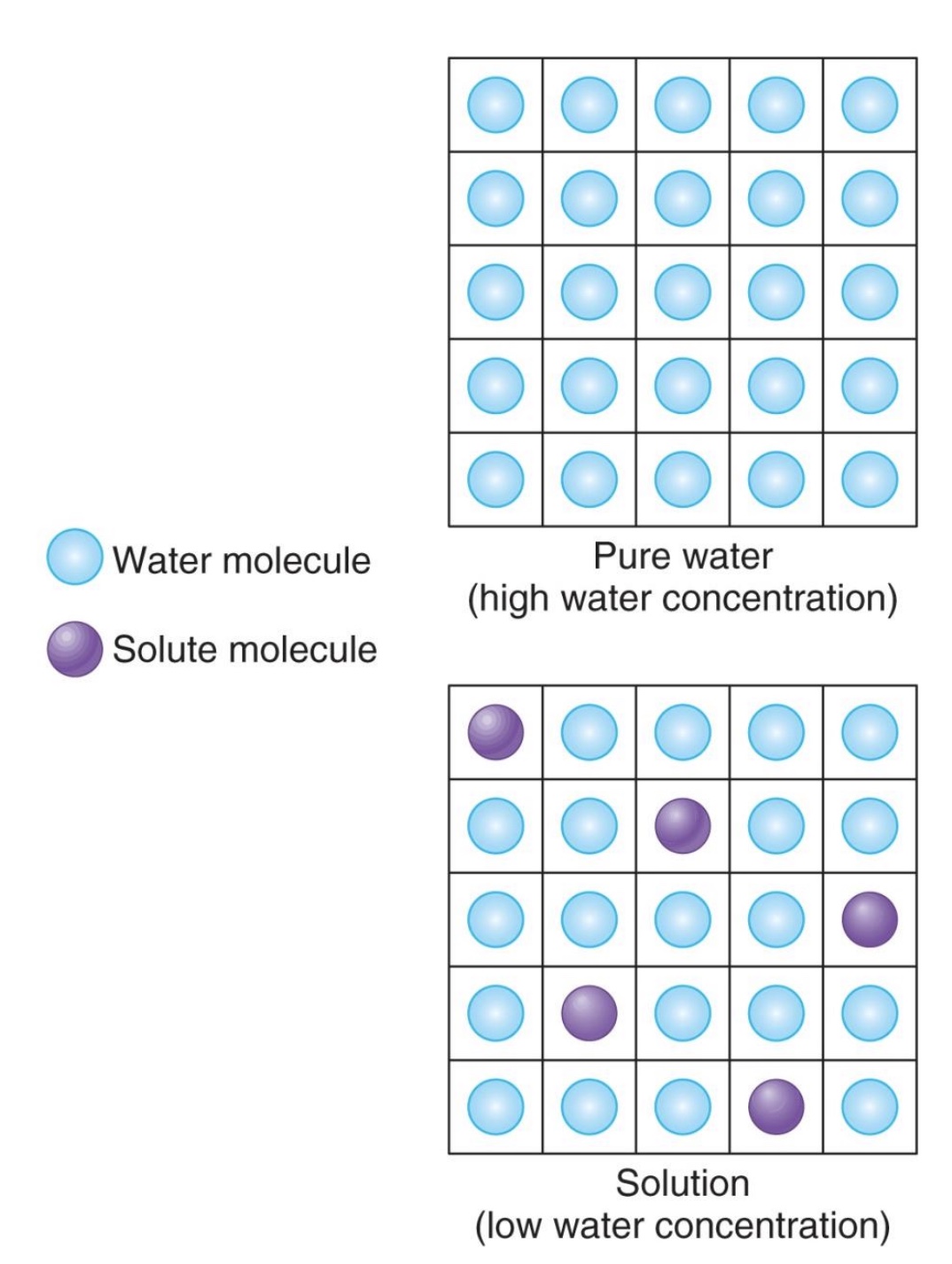
pure water vs. water with solutes
-solutes take up space so have lower water concentration than pure water
why we care about osmolarity and tonicity
-Homeostasis: the maintenance of fluid volume and electrolyte concentration is essential for normal body function and survival
-In medical settings, it is common to give intravenous fluids (IV’s)
The wrong choice of fluid carries significant risk of patient harm; Certain fluids can cause cells to swell or shrink, depending on what is dissolved in the fluid
Example complication: brain swelling
*saline= 0.9% NaCl = same as plasma and ICF; want to give patient an isotonic solution
osmolarity (+relation to molarity)
-A measure of concentration
Defined as the total amount of dissolved particles per liter of solution
Expressed as osmoles per liter (osmol/L or OsM);1 osmol is one mole of particles
-Related to molarity, but not necessarily the same – depends on dissociation
-For substances that do not dissociate in solution: same (*ex: glucose)
-For substances that do dissociate in solution:
Form multiple particles
Example: 1 mol NaCl dissociates to form 1 mol Na+ and 1 mol Cl- . A 1 molar NaCl solution has an osmolarity of 2 osmol/L (assuming full dissociation)
-The osmolarity of a solution does not tell us whether it will affect cell volume – we need to know what is dissolved, in addition to the quantity
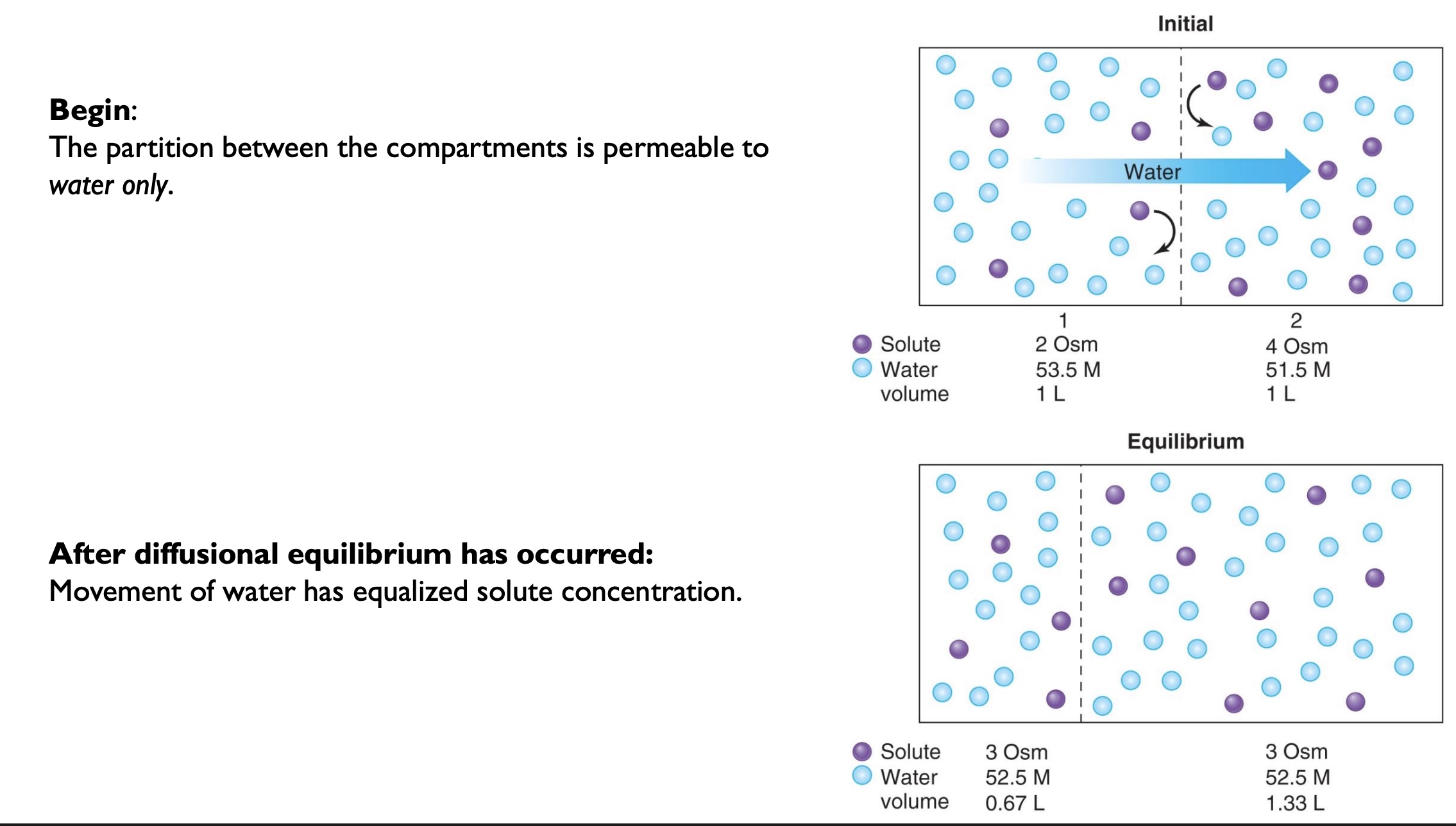
-are most membranes semi-permeable, fully permeable, or non-permeable?
-what is a limit of biological systems when it comes to osmosis?
-semi-permeable
-membranes have limited ability to change volume
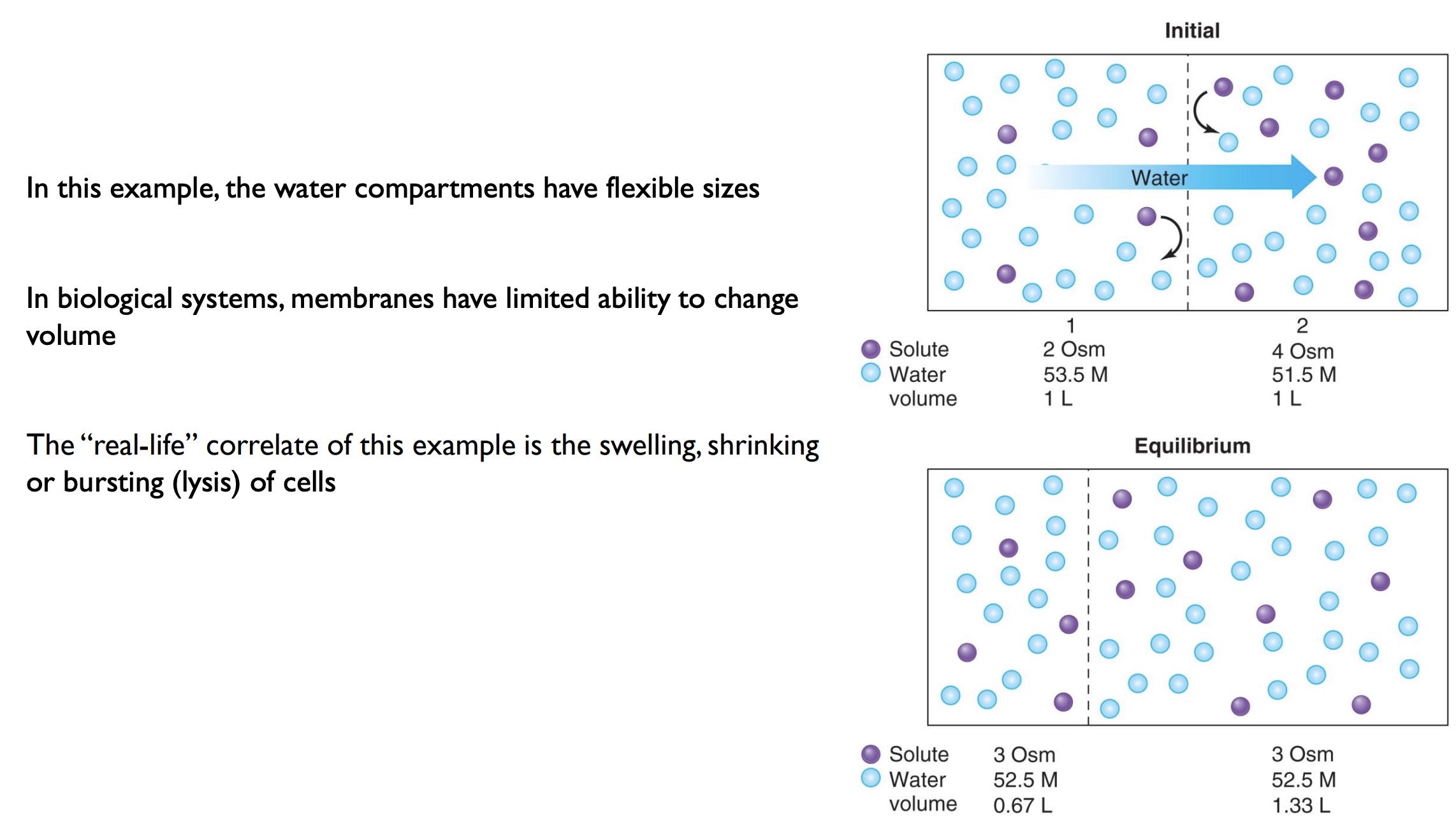
nonpentrating solutes
-These substances that cannot cross the membrane without assistance are called nonpenetrating solutes
-Sodium functions as a nonpenetrating solute because it is pumped out of a cell as rapidly as it enters (Na+ stays on the outside of a cell)
tonicity (definition, when relevent, what determined by, etc.)
-A description of how a solution affects cell volume (swelling or shrinking)
-Relevant where two compartments are separated by a semipermeable membrane (e.g. extracellular and intracellular fluid)
Penetrating solutes can freely diffuse across the membrane
Nonpenetrating solutes cannot freely diffuse across the membrane
Some cannot cross at all
Others diffuse across the membrane but are rapidly transported back in the opposite direction – e.g. a small amount of Na+ leaks into cells, but it is removed by the Na+ /K+ -ATPase
-The concentration of nonpenetrating solutes is what determines tonicity – water moves by osmosis toward the compartment with a higher concentration of nonpenetrating solutes
what is the osmolarity of ctysol?
~300 mOsm/L
*saline=0.9% NaCl and is also the same
hypertonic vs isotonic vs hypotonic
-hypertonic: water moves out of the cell, and the cell shrinks/”crenation”
-isotonic: concentration dissolved outside=inside, no net movement of water
-hypotonic: water moves into the cell, and the cell swells and could potenentilly burst
*ex: if drink distilled/plain water
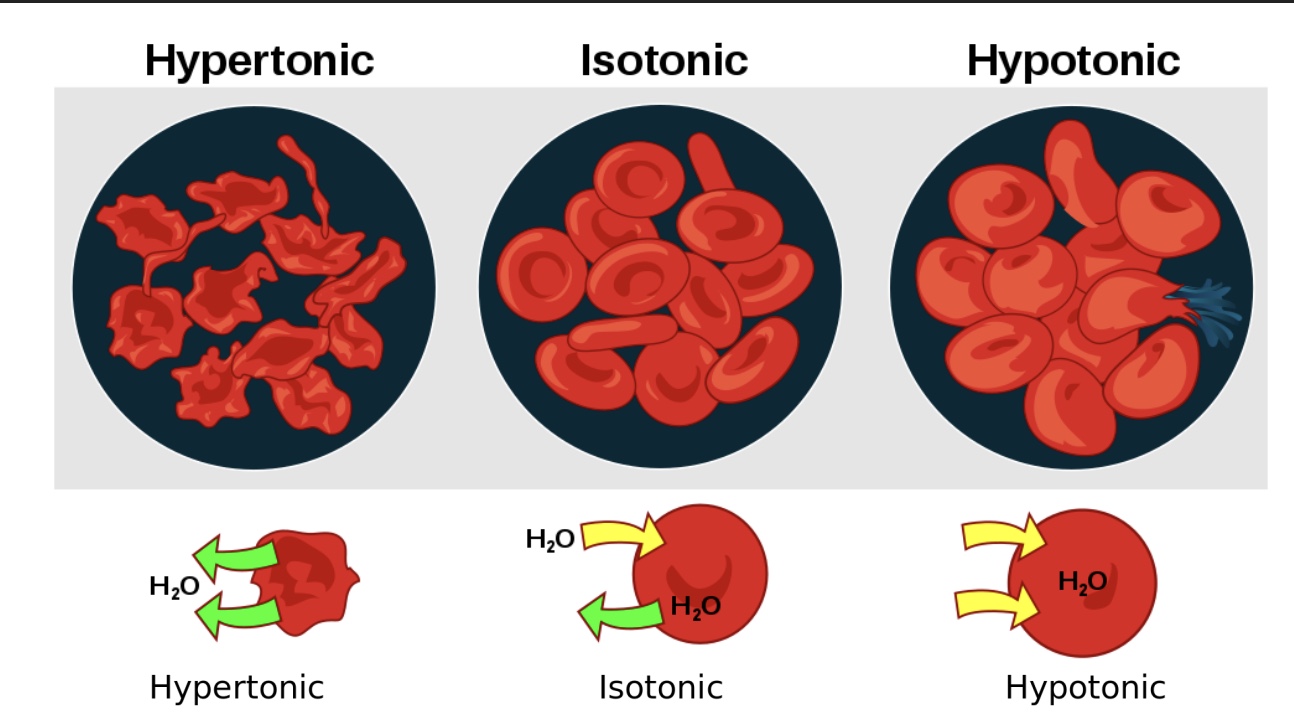
true or false: osmolarity and tonicity are the same thing
false!
-osmolarity=concentration
-tonicity=how cell volume changes
Hypernatremia and Hyponataremia clinical conditions
-Hypernatremia=too much Na+
diabetes insipidus: produce dilute urine → large water loss (dif than DM)
loss of hypotonic fluids (sweating, vomiting)
-Hyponatremia
excess water consumption
adrenal insufficiency (Addison’s disease): adrenal glands make aldosterone which keeps Na+ in the body become insufficent
*rare to get though diet, medical more common
numerous iatrogenic causes (medical interventions) for both of the above (drugs, infusions of wrong osmolarity solution, etc.)
Correcting Na+ levels
-Done slowly and cautiously (allow body to gradually recalibrate)
-Example: hypernatremia should not be corrected by rapidly infusing sterile, deionized water (0 mOsm)
-Safer treatment would be half-normal saline (0.45%) over hours to days
*ex: Brain swelling, excess water puts pressure on brainstem
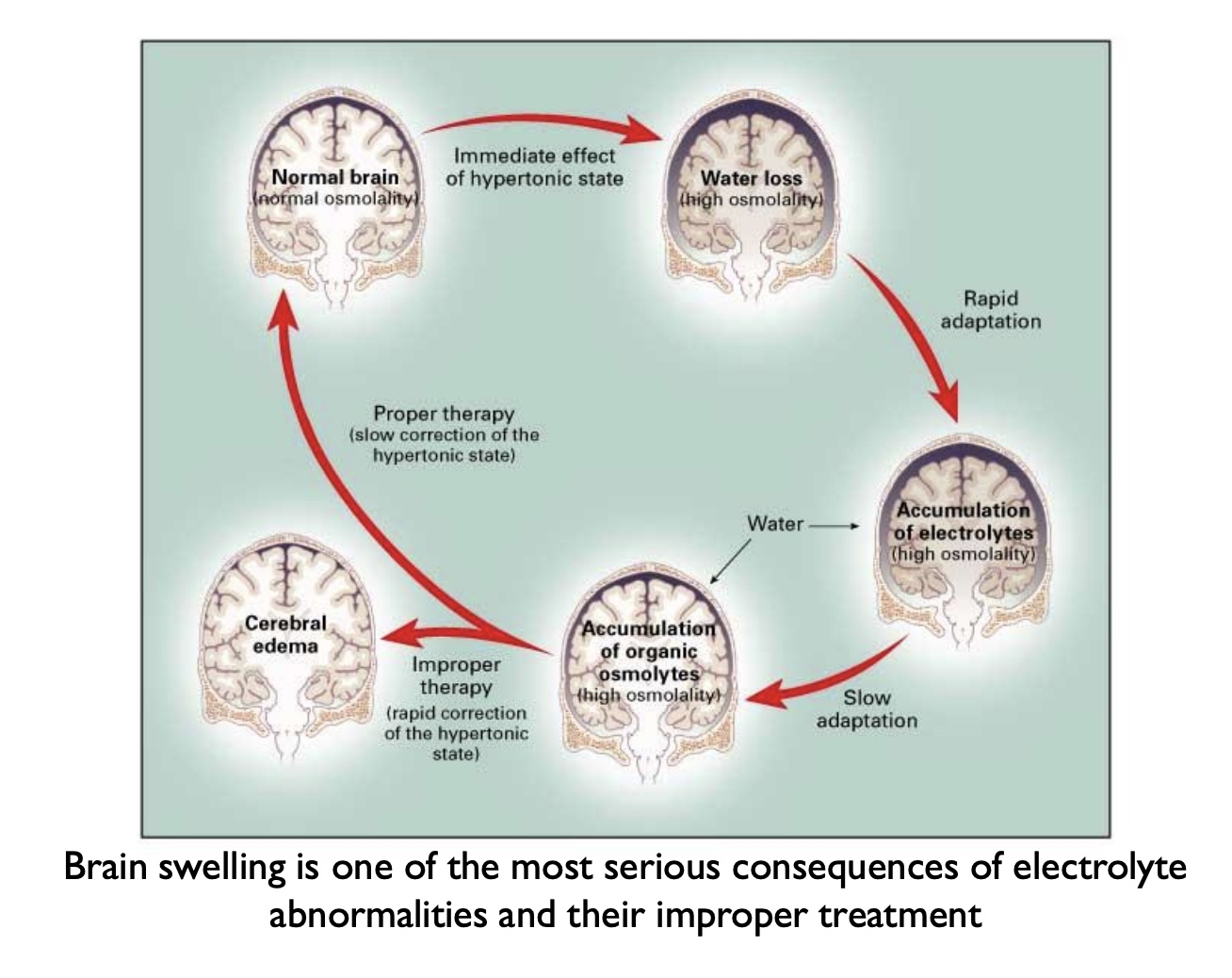
Homeostasis in terms of osmolarity
-Specialized neurons detect osmolarity and can regulate thirst and salt/water excretion: Hypothalamus “Osmoreceptors” secrete hormones that signal for water retention or can stimulate thirst
-The kidney is the main effector organ for these responses (adjust urine osmolarity)
-Urine can range from <100 - 1400 mOsm
*1400=limit, if not enough water
-”Obligitory Water Loss”: kidney gets rid of water w/ Na+, low BP and kidney’s shut down
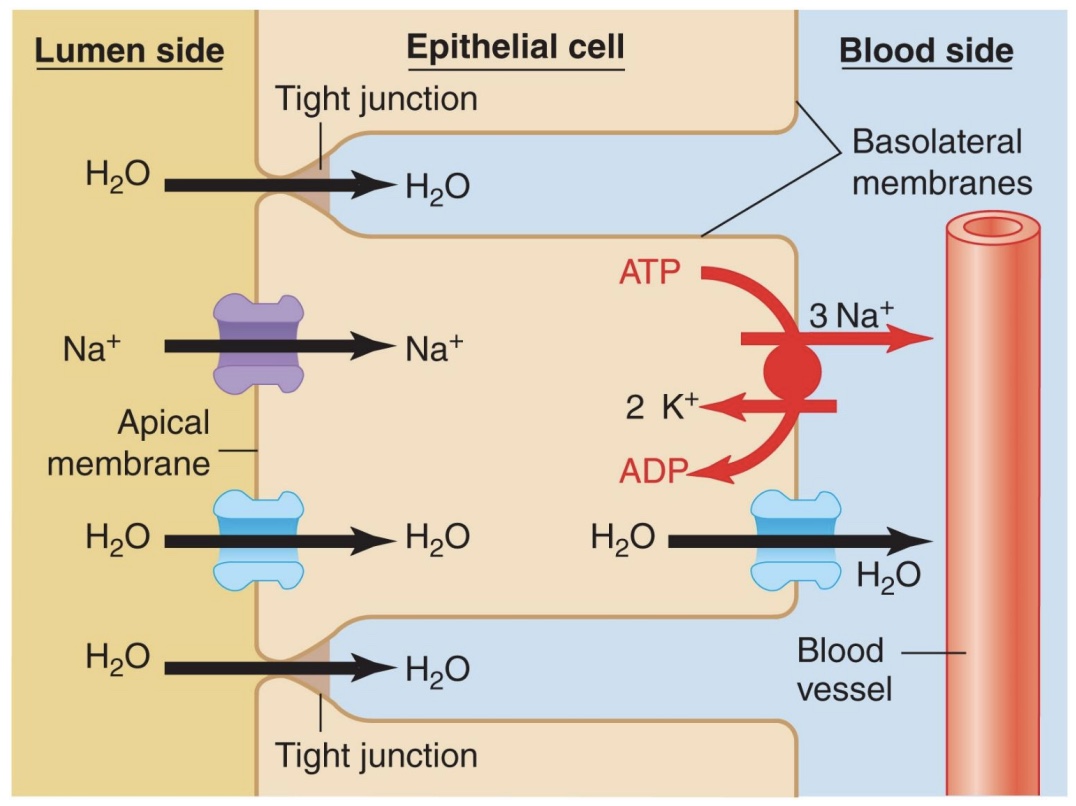
Na+ transport mechanisms in the kidney
-Intracellular Na+ concentration kept low by active pumping; This allows diffusion of Na+ from lumen into cell; Net uphill movement
*nephron does fine-tuning
-ATP pump powers counter and co-transport, ex: H+; ex: transports glucose
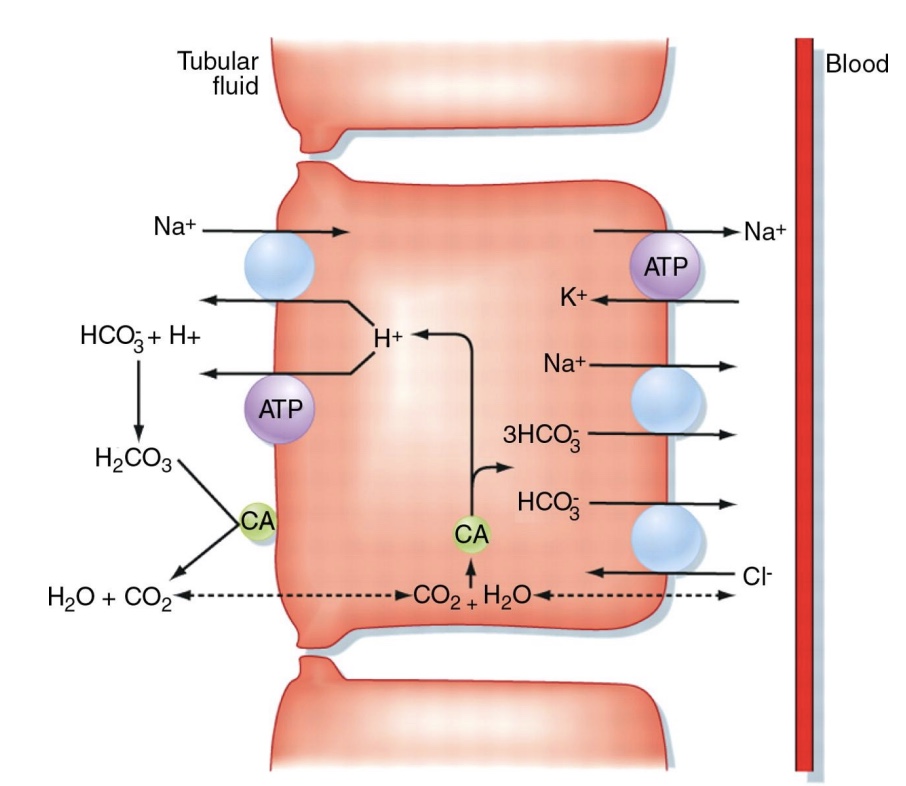
Movement of water lumen side → blood side in kidneys
-Moves by osmosis toward higher sodium concentration (lower water conc.)
-Can go through channels (aquaporins)
enhance membrane permeability to water, though some water can go though without
hormone regulated
-Is one of few substances that can cross tight junction