Introduction to diffraction and X-ray crystallography
1/239
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
240 Terms
what are spectroscopic techniques?
study the environment of atoms of the molecule we are in, and use this to deduce structural information
Name some spectroscopic techniques
CD, UV
FTIR
NMR
XAFS
What are scattering techniques?
use scattering to form an image of the molecule → a direct visualisation
Name some scattering techniques
Dynamic laser light scattering
SAXS
Microscopy
Neutron scattering
X-ray diffraction
What is structure prediction?
predicts likey structure from a protein from its sequence using neural networks, rather than experimental data
useful, not biophysical
Alphafold2
Rosettafold
What is the basic idea of NMR?
Based on some atomic nuclei having a magnetic moment / spin, and the way these spin in the presence of different environment can be used to probe their environments
Multi-dimensional spectra allow the calculation of distances between nuclei both through space and through bonds
This allows the calculation of a set of distance constraints that must be obeyed by any molecular structure compatible with the spectra
This will be repeated many times to generate an ensemble of structures consistent with NMR spectra
we get highly conserved regions - always look the same
we get regions that vary - all are compatible with experimental observations
more dynamic observation
What sort of results does NMR give?
Structures of small proteins (less than 40kDa)
Studies of flexible systems (and how well defined domains relate to eachother and moce with respect to eachother)
Solvent and temperature effects
Site-specific screening (can observe site specific modifactions / ligands)
useful in drug design
What is SAXS?
small angle x-ray scattering
Describe SAXS
Scattering technique - not used for high resolution structures
Solution method rather than a non biological environment
SAXS collects scattered X-rays from molecules in solution.
These are assumed to be randomly oriented and so scattered x rays are radially symmetrical around the direct beam
Desrcibe the results of SAXS
Produces an intensity curve - falls off with resolution
but this is not linear
This volume represents an ensemble of the shapes adopted by the molecule in solution
from the images, we can calculate a distribution of pairs of atoms within the structure
in the plot, large distribution that are 20A from eachother - this data suggests a rather elongated molecule with a dense region at one end
Volume calculation is carried out many times and an average calculated – final result could be considered an ensemble of ensembles
Can be useful with flexible systems
can give information about how domains within overall flexible system are in respect to eachother
What does CryoEM use?
Vitrified samples exposed to electrons
What does vitrified mean?
vitrified - flash cooled at liquid nitrogen temperatures (no ice present)
How does CroEM work?
Vitrified samples exposed to electrons
vitrified - flash cooled at liquid nitrogen temperatures (no ice present)
Images reconstructed using magnetic field as lens
Technological developments in e-detectors mean Cryo-EM is now an atomic resolution technique for biological samples
approaching atomic resolution is routine
now the primary method used to determine macromolecular structures
Why is crystallography a good method for structure determination?
High resolution can be achieved
can be routinely carried out rapidly if a similar structure has already been solved → implications: if trying to develop drugs or measure small molecule binding against a protein system
We want to look at objects on the scale of atoms to large molecules → X rays are ideal for this
What is the best thing about crystallography?
Possible to study atomic details of structures from a few atoms up to large macromolecular assemblies → only system that can do this
What can be difficult with crystallography?
difficult to grow good crystals of larger structures → so although they ave been solved by crystallography, we can now use electron microcopy
Why dostructural biology?
Study structure function relationships
Possible to gain insight into mechanisms of action
Haemoglobin structure (Perutz) reveals mechanism of oxygen storage
Ribosome structures (Ramakrishnan, Steitz, Yonath) reveal much about the mechanism of protein synthesis
How can conformations be derived form crystallography and EM?
Although both crystallography and EM both take static snapshots (rather than recording a dynanic nature of molecule in vivo), taking many snapshots can give information about molecular states
Is the crystalline state used in crystallgraphy an issue?
Generally no!
Many enzymes are active in crystalline state (eg. DNA polymerase)
Crystal structures generally agree well with measurements from spectroscopic techniques (eg. NMR, fourier transform IR, fluorescence)
rare to significantly diverge from solution based methods
generally - the crystalline state doesnt introduce large artefacts
Describe how protein packing in crystals doesnt actually affect structure
Proteins are often observed in more than one “packing” in different crystal forms: generally the differences are either slight (~0.1 Å), or important (e.g. multiple conformational states)
usually - the structure is the same
we see 2 well defined different states - useful
Protein crystals are typically > 30% solvent, and less than 30% of a protein surface is involved in crystal contacts
in crystals, average 50% solvent, so most of the surface are interacting with water
theres only a few points of the protein making contacts
What sort of concentration are protein crystals?
very high concentration solutions (typically ~10 mM)
What are the main processes in crystallography?
Expression/purification
Crystallisation → need to grow crystals
Data collection → by exposing toX-rays
Map calculation → exposing to electron density maps
Map interpretation → interpreting the electron density map
What do we need too form a crystal and why?
Need a saturated solution (and super saturate it) → so the salt comes out of solution, slowly
Why can’t we use heating and cooling for protein crystallisation?
proteins will denature and wont form a crystal
What is the main method of protein crystallisation>
Vapor diffusion - this allows the concentration of protein in a drop to gradually increase
What are the different methods of vapour diffusion>
hanging or sitting drops, drop of solution under oil (gradual evaporation of water through the oil layer), small scale batch dialysis
What are the variables that can vary our ability to crystallise?
Temperature
pH
Precipitant - choice of and concentration
adding different chemicals that alter protein solubility as concentration changes
protein concentration
Salt
Detergent
Ligands
may bind the protein and facilitate crystallisation - eg. aid contact formation
→ many variables, difficult to screen systematically, most large groups rely on automation and brute force
What is on the X and Y axis for the solubility phase diagram?
Y axis = conc of protien of interest X axis = crystallant concentration (we hope this will reduce the solubility - historically a salt (Ammonium sulfate), now - polyethylene glycol
Describe the 3 main defined zones of a crystal phase diagram
clear protein solution → single phase, protein is soluble. solubility line is the boundary of this stable soluble state
metastable soluiton → possible for the protein to separate into soluble protein (saturated solution) and some that has come out of solution.
how this behaves depends on other conditions
will have a boundary - on one side there is a possibility of spontaneous nucleation (crystallisation) and a groeth zoen (can still come out of solution but dont crystallise)
precipitate plus protein two phase
what occurs to proteins in the clear protein solution stage?
single phase, protein is soluble. solubility line is the boundary of this stable soluble state
what occurs to proteins in the metastable solution stage?
possible for the protein to separate into soluble protein (saturated solution) and some that has come out of solution.
how this behaves depends on other conditions
will have a boundary - on one side there is a possibility of spontaneous nucleation (crystallisation) and a growth zone (can still come out of solution but dont crystallise)
what occurs to proteins in the two phase stage?
protein aggregates and comes out of solution
Describe the process of crystallisation in vapour diffusion
Put drop of protein on coverslip
Add a drop of crystallant solution - both are at half their original concentration
Fix this on a resevoir above a solution
Vapor pressure in the sealed environment is dominated by the reservoir
osmotic effects mean that concentration in the droplet are driven to equilibrate wit the concentration in the reservoir
water leaves until the concentration of crystallant is equal to that in the reservoir
leads to equilibration
should take us into the spontaneous nucleation zone, and so we get crystals grow
protein concentration drops because coming out of solution
What can go wrong in crystallisation?
Over nucleation
Spontaneous precipitation
Non-crystalline phase separation
Failure to reach super saturation
What is over nucleation?
can get too many nuclei and not have enough protein left to form individual crystals
What is spontaneous precipitation?
everything aggregated out - need a new condition
What is non-crystalline case separation?
protein has come out of solution but not nucleated
sign you are approaching a useful condition, need to stimulate nucleation
What is failure to reach super saturation?
protein is so soluble, and so it stays in solution
can usually find a way to drive it to precipitation
What is the basics of forming a crystal?
copies of a simple component (the unit cell) are packed together by translation to fill space.
This packing must be infinitely extensible in all directions
no sudden boundaries
What is a plane lattice?
A plane lattice is an infinitely extensible construct of intersecting parallel and equidistant lines, forming identical unit lattices.
have an origin, a square unit lattice defined by vectors a and b (have magnitude and direction, defining their direction of movement from the orign. → these have an angle between them (gamma) a = b, and gamma = 90
this describes the square lattice too
What is a unit cell?
what is repeated to make a crystal
What does a unit cell consist of?
nit lattice - described by vectors
Motif - the actual content of the unit lattice
Describe how origins can be different in different crystals
As long as each unit cell contains the same integer number of molecules, this is fine - doesnt have to be one in one - could be spread ver multiple
Describe what a symmetry operation must do in a crystal
Applying a symmetry operation to cannot generate any changes in a motif
the protein has to stay the same
for chiral molecules such as proteins, this rules out any inversions or mirror operations → we cant have any mirror images
What are the different symmetry operations in the unit cell?
Translational symmetry
Rotational symmetry
Describe rotational symmetry
Axis of rotation - A rotation can only involve operations by (360/n)° → in between each copies, you wont see any partial copies
ll symmetry operations must also be compatible with the translational requirements of a given lattice
as a consequence, only 2-, 3-, 4- and 6-fold rotations are allowed and are indicated in the International Tables for Crystallography by dyad, triad, tetrad and hexad symbols
Rotational symmetry must be compatible with lattice symmetry. eg. a square plane lattice is not compatible with a 3-fold rotation
Describe the 3D symmetry of crystals
molecule in a unit cell is packed together in space, and these form a crystal
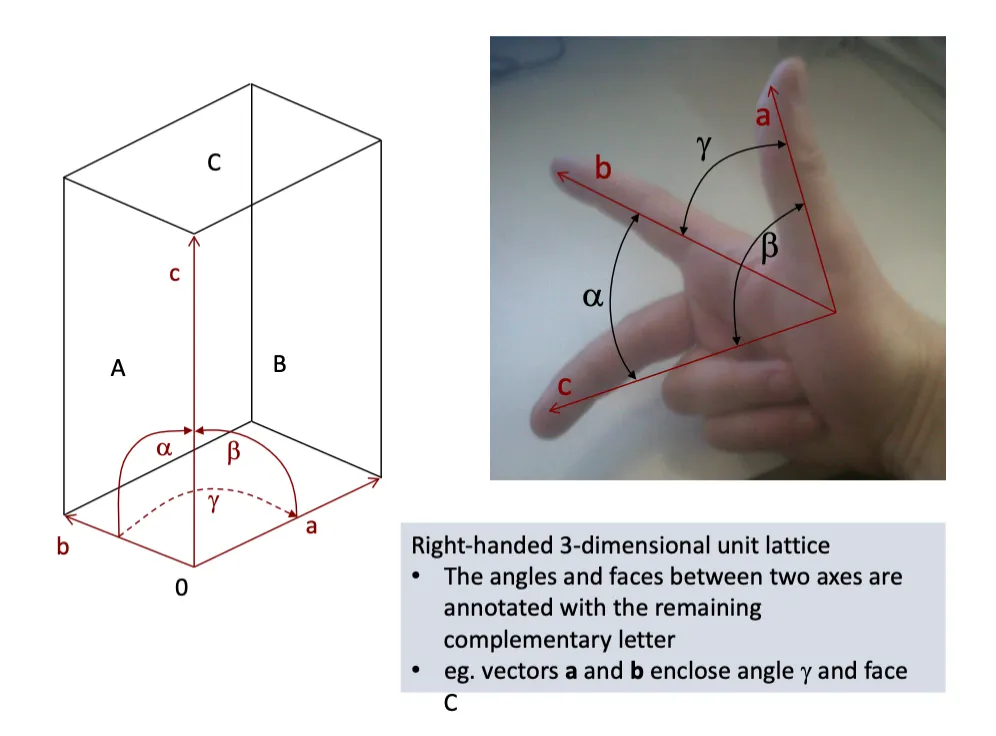
What is a right handed unit lattice?
right handed , 3D lattice
Can use the right hand to show the relationship between a b and c
Whatever the missing letter is is the angle between the two
What is a primitive lattice?
one lattice point per unit cell
What is a lattice point?
Lattice points are all connected to other unit cells through translation
What are the symmetry contrasts of a primitive lattice?
If a lattice shows 4 fold rotational symmetry, all unit cell angles must be 90º → consisitent with tetragonal or cubic systems
Restricts the possible symmetry elements
What is a centred lattice?
contain two or more lattice points per cell
When is a cantered lattice possible?
Possible when unit cells interleave with eachother
this means each unit cell contains additional lattice points
so there are regions of the unit cell that are obliged to be symmetrical to eachother even thouhg there is no rotation
higher symmetry since for a centred cell to exist, some regions of it must be identical to corresponding (but not spatially identical) regions of adjacent cells.
What is a C centred lattice?
Centered in the C face
What is an F centred lattice?
Centeref in all faces
What is an I entered lattice?
A body entered lattice at the centre of the unit cell
What are screw axis (roto-translation)
Combines rotation and translation along the axis
produces a helical arrangement in the crystal
Forming a helix passing through a crystal
In two fold symmetry - If we move both 180º and moe up half the height of the unit cell, we have arrived at the same poistion in the next unit cell → so we form a helix
What are the general bits about symmetry?
65 possible space groups for proteins - 230 if things can be non-chiral
Large scale of combinations
Main thing: unit cells repeat translationally, within the unit cell, all othe symmetry is assymetruic units related to eachother by symmetry
Symmetry in the crystal lattice is paralleled by symmetry in the diffraction pattern
What are Xray sources?
Synchrotron sources
When are synchrotron radiation emiited
Synchrotron radiation is emitted by charged particles travelling at relativistic speeds when they accelerate.
In a synchrotron this involves electrons being steered around a curved path by magnetic fields.
accelerate towards the centre of a circle, and as a result it emits radiation
Synchrotron radiation is generated whenever the electron beam changes direction.
What can synchrotrons emit?
Broad, continuous spectrum from microwaves to hard X-rays is produced.
Describe the elements of a synchrotron storage ring
Bending magnets - cause the electron beam to bend → we can also make it bend multiple times as it moves past different pairs of magnets - undulators
If the electron beam is passed through an array of magnets with alternating polarity, SR is generated at each bend in the e-path.
bend particles round corners to close the orbit and give rise to SR
Electrons pass through these magnets and are deflected from their straight path by several degrees. This change in direction causes them to emit synchrotron radiation
Focusing magnetic structures
quadrupoles, sextupoles, no SR
Periodic magnetic structures
wigglers, undulators
What is the function of a goniometer>
device for precisely fixing a sample - can rotate it and transalte it
What are the traditional X ray detectors? What are the issues
photographic film
issues: slow, labour intensive, low dynamic range
What are the newer X ray detectors?
CCDs and pixel counting detectors
What is good about CCDs?
charge coupled devide
fast, low noise, high dynamic range
What is good about pixel counting detectors?
new technology, each pixel counts photon events independently
so fast they have changed the nature of data collection
constant roation, no more stopping for detector readout
very high dynamic range
Describe how data is collected in diffraction (basic_
We have a crystal that we rotate - this is because we can only sample a small fraction of spots in a given orientation
Set crystal to detector distance (resolution)
Centre crystal in beam set crystal to detector distance
Test diffration quality at 0, 90º
Determine data collection strategy
Set up data collection
Collect data
What is grid scanning?
crystal mount is translated and short exposures are taken of many areas of the sample.
How do we form an image in diffraction techniques, what is the difference with X-rays?
An image is formed by
exposing sample to radiation
radiation is scattered
a lens (in EM) recombines these scattered waves
the final image depends on all of the diffracted rays
For X-ray, there is no lens, and so wave recombination is done computaitonally
What radiation do we consider in diffraction?
We only consider elastically scattered radiation → photons that do not lose any energy
What are the requirements for diffractive techniques?
Must use radiation with wavelength approximately the size of details to be observed (atomic bonds are approx. 1.5Å in length) therefore electrons, X-rays or neutrons
Only “elastically scattered” radiation considered – we ignore the contribution of photons that lose some energy to the scattering object
Combination achieved either by focusing (EM, optical microscopy) or computationally (X-ray or neutron diffraction)
Each pixel of the reconstructed image depends on all of the diffracted waves
What is a Fourier syntehsis?
representation of a complex function in terms of a set of sine waves
Describe the main point of a Fourier synthesis
solving a structure by X ray crystallography is a process of adding waves together
we need some way to treat and consider waves
way of representing a periodic function (electron density in a crystal) as a set of sine waves
Why is a Fourier synthesis useful - why does it work?
adding waves with different frequencies, amplitudes and phases allows for a good approximation of atomic distribution
The fourier transform of the unit cell gives a set of peaks, ith the large peaks corresponding to the most important frequencies
These correspond exactly to the sine-wave frequencies used to reconstruct the unit cell. The peak height also corresponds to the sine-wave amplitudes
What information do we lose / gain when we do a Fourier transform?
we lose information amount the phase of the waves, but gain information about frequency and amplitude
What gives rise to the position of diffracted spots in X-ray crystallography?
Size and shape of the unit cell
What gives rise to the intensity and phase of the diffraction pattern in crystallography?
The molecules structure
Explain why atoms scatter X rays
Dealing with an electromagnetic wave and its interaction with charged species (electrons mostly)
This makes it oscillate, and these acts as new sources
When multiple electrons are emitting, scattered waves interfere with eachother, producing a non cylindrical symmetric pattern
Where there is more than one such piece of charge, the intensity of the scattered beam is prone to interference.
Scattered intensity in any direction depends on the entire charge distrubution
Each point in the reconstructed image depends on all recorded diffraction
When do waves scatter in phase?
When the path length is the same for a given 2 waves
leads to positive interference between the two waves
When the path lengths differ by nλ
integer multiple of the wavelength of the radiation
this is braggs law λ=2dsin0
not reflecting - x rays are passing through the crystal and being scattered at a specific angle (theta), and so we see positive interference
How are lattice planes defined?
defined by how they intersect the unit cell axes
What are the different miller indices to describe planes?
h - how many times a plane crosses the a axis
k - how many times a plane crosses the b axis
l - how many times a plane crosses the c axis
must be integers - only this satisfies the constructive interference conditions
What do larger miller indices mean?
more times the planes cross the axis of the unit cell, finer set of resolutions
planes are closer together
smaller d spacing
higher scattering
higher resolution
What is a reciprocal lattice?
considering the family of diffracted reflections from a crystal
Why can we consider the reciprocal lattice as so?
There are sets of planes that only diffract if they cut each of the cell edges in in integral number of times
this is because these are the only ones that give rise to constructive interference
We can therefore imagine a conceptual grid of reflections related to the geometry of the repeating unit cell of the crystal.
reciprocal lattice - considering the family of diffracted reflections from a crystal
What dies a larger unit cell mean for real space and reciprocal lattice points
Larger the unit cell - the smaller the difference between bragg angles for neighbouring sets of planes in a lattice and the smaller the spacing between reciprocal lattice points
large unit cell results in a small separation in reciprocal space
What does each property mean on an argand diagram?
Waves to add together can be characterised by amplitude and phase
Waves as a vector
amplitude = magnitude
phase = angle of rotation around a complex plane
How do we add up waves in an argand diagram?
We can represent waves as vectors, and add them by drawing them nose to tail
How does addition of atomic scattering vectors produce a structure factor?
Each atom contributes a scattering vector upon interaction with X rays
The phase depends on the atoms pposition relative to lattice planes
The structure factor is the sum of all atomic contributions
and so is dependent on the arrangement of atoms in the unit celll
To work out the contribution of all of the waves, we draw a vector from the start of the first to the end of the last
this is a structure factor - dependent on the arrangement or structure of atoms in the unit cell
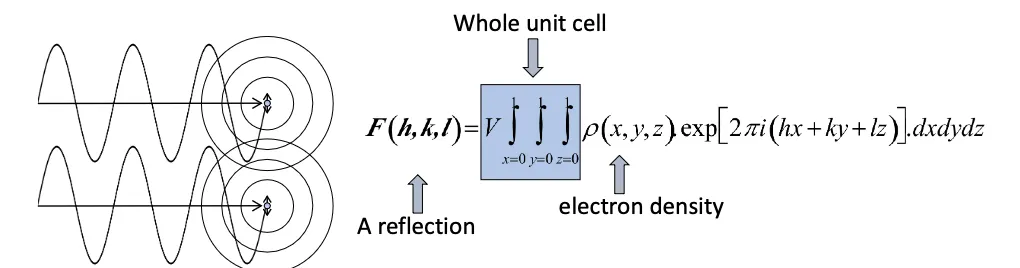
What is a structure factor?
a mathematical function describing the amplitude and phase of a wave diffracted from crystal lattice planes characterized by Miller indices h,k,l.
WHat is the structure factor equation?
What do we aim to get from structure factors?
we need to calculate electron density from structure factors
What are the principle begins the electron density eqution?
Real space function
A lens will bend the scattered light, applying a wave shift and reforming an image
Structure factors are in reciprocal space
Electron density - the electron density at a point in real space
The electron desnsity is obtained by the inverse fourier transform
Each point on the map is a sum of may waves with different phases and amplitudes
Normally a lens will
Bend the scattered light pattern
Apply a phase shift
Each “pixel” of the image formed is therefore a sum of many scattered waves. These will add up in a way that depends upon their phase and on the position of the pixel.
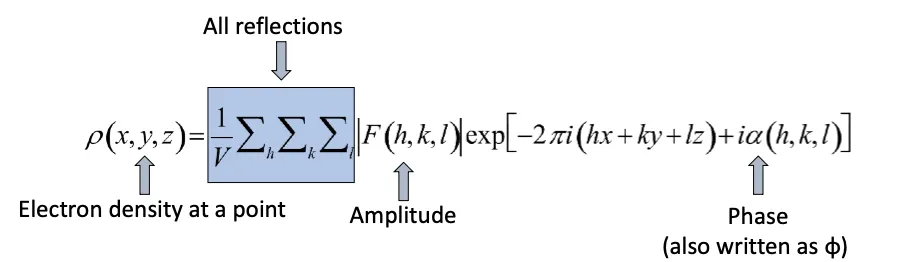
What are the components of the electron density equation?
contains terms for the amplitde with a set of miller indicis and the phase
has a phase term, amplitude term and electron density is in real space
What does a lens do?
Bend the scattered light pattern
Apply a phase shift
What is the electron density equation?
What is convolution?
take advantage of the understanding of the waves as Fourier phenomena
What are the two theories of convolution?
Convolution theorem
Inversion theorem
What is the convolution theorem?
Convolution of two functions mixes them together. For functions f and g this can be expressed by the formula:
If f is the motif (protein of interest) and g is a lattice (crystal lattice), both are described as funcitons
The convolution of these two is f*g - there is a copy of the molecule at every point in the lattice
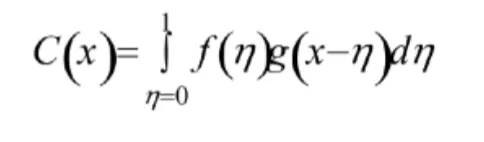
What is the basic explanation of the convolution theorem?
The convolution theorem states that the Fourier transform of the convolution of two functions is equal to the product of the Fourier transforms of the two separate functions