Learning outcomes Physical and Physiochemical aspects of food technology
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
55 Terms
Function of mesoscopic physics
It acts as a bridge for formulating relationships between properties on a molecular scale and a macroscopic scale.
Categorizations (10 different ones)
Milk and dairy
Eggs & egg-based products
Meat
Fruits, vegetables and herbs
Grains and nuts
Bread and dough
Sauces
Confectionaries
Alcoholic beverages
Drinks and juices
Two issues with formulating relations between physical properties on a molecular scale and properties on a consumer relevant scale
Difference in lengthscale of a factor of a billion
Structural inhomogeneity
Mesoscopic structures
A structure that has a size between 1 nm and 1 mm
Colloidal systems
Consist of small particles dispersed in a continous medium
Something that falls in between a homogenous mixture and a heterogenous mixture
For example Fog is a colloid.
It consists of very tiny droplets of water dispersed in air
The dispersed phase are the water droplets
The continuous phase is the air where the water droplets are found
What type of flexible structure can result in the rigidity of its system with a low amount of mass?
Platelet-built structure
Order in different states of matter (solids)
Molecules are arranged in a fixed, regular pattern.
If you plot the number of molecules at these specific distances, you will observe "peaks" in a graph indicating that there are more molecules present at those discrete distances. The peaks reflect the structural regularity and periodicity typical of solid materials.
Order in different states of matter (liquids)
The arrangement is less rigid than in solids, yet there are still clusters of correlated molecules.
This limited extent of order indicates a more disordered and fluid molecular arrangement compared to solids. The peaks in a graph would still exist but would be much less pronounced and only noticeable over short distances, highlighting the transient nature of the interactions.
Order in different states of matter (gases)
There is no order, molecules are far apart from each other and move freely
In this state, you'd expect the graph depicting molecular distances to show no significant peaks, indicating a lack of consistent molecular arrangement or correlation at any distance
Relative humidity formula
p/pmax = RH
p=water vapour pressure
pmax=the maximum water vapour pressure
Water activity (aw)
Pproduct/Pmax
Pproduct=The water vapour pressure of the product
Pmax=The maximum water vapour pressure
Name 3 principle parameters that affect the physical properties of a system
Concentration, temperature, and pressure
Structure of milk
Fat globules, embedded in a fluid.
On a smaller scale one has casein micelles
These are built up of various proteins, among other constituents.
Whey proteins: These proteins, dispersed in the liquid surrounding the fat globules and casein micelles, contribute to the nutritional value and also play a role in the structure of dairy products.
Diameter of a fat globule
Range of 1 and 5 micrometers.
What is the thin shell of a fat globule made out of & what is its purpose?
Proteins
Phospholipids
Vitamin A
Cholesterol
These prevent the globules from coalescing together
Diameter casein micelle and what does it consist of?
Diameter is 0.1 microns
Consists of several types of protein aggregated together
Contains other consituents like calcium and phosphate ions, forming calcium phosphate complexes
What are the hairy regions in casein micelle made of and what is its function?
Formed by kappa-casein
These prevent the casein micelles to coagulate together and limit the casein size.
Whey proteins
The class of proteins that are most relevant from a point of view for structure purposes
they are present in the aqueous fluid surrounding the casein micelles and fat globules
What are the four basic whey proteins
Alpha lactoglobulin
beta lactoglobulin
Bovine serum albumin (BSA)
Immunoglobulin
What does the rate of creaming depend on?
The properties of the fluid surrounding the particle
If one has syrup, particles cream slower than when the fluid is water (viscosity)
Parameter viscosity of a material
A measure of the difficulty to induce a flow of the material.
Higher viscosity = more difficult to induce flow, lower the creaming rate of the single fat globules
Colloidal characteristics of mayonnaise
Continuous phase: Water and vinegar or lemon juice
Dispersed phase: Oil
Colloidal characteristics of Dough
Continuous phase: Water
Dispersed Phase: Proteins (gluten), starch, and fats
Colloidal characteristics of Whipped cream
Continous phase: Air
Dispersed phase: Cream (fat globules suspended in water)
Colloidal characteristics of margarine
Continous phase: Fat
Dispersed phase: Water, typically around 15-20%
Colloidal characteristics of milk
Continous phase: Water
Dispersed phase: Fat globules, proteins (casein and whey), lactose
Colloidal characteristics of Homogenized milk
Continous phase: water
Dispersed phase: Smaller, more uniformly distributed fat globules
Colloidal characteristics of butter
Continuous phase: fat
Dispersed phase: water
Colloidal characteristics of yogurt
continous phase: water
Dispersed phase: milk proteins (casein) coagulated, containing fat and live bacteria.
The spreadability of mayonnaise in terms of physical macroscopic property
Viscosity: Mayonnaise has a moderate viscosity, which allows it to flow easily without being too runny.
Texture: The creamy and smooth texture contributes to a pleasant mouthfeel and enhances the interaction between mayonnaise and the spreading tool
The spreadability of mayonnaise in terms of colloidal scale
The size and distribution of these droplets are crucial for spreadability.
Smaller, uniformly distributed droplets create a more stable emulsion, leading to improved spreadability.
This is because smaller droplets have a greater surface area, allowing emulsifying agents, like lecithin from egg yolk, to more effectively coat them and prevent them from coalescing.
The spreadability of mayonnaise in terms of molecular scale characteristics
Emulsifying agents:
Molecules such as lecithin (from egg yolk), are amphipathic.
This allows them to stabilize the oil-water interface, preventing separation and contributing to the creamy texture that makes it easy to spread.
Molecular interactions:
Hydrogen bonding and van der Waals forces between water molecules and emulsifying agents help maintain the emulsion's stability.
Plasticity (adaptibility):
The arrangement of fat molecules, especially the length of their fatty acid chains, influences how easily a product deforms and flows, contributing to properties like stiffness and spreadability.
Colloidal structure milk & homogenized milk
Milk:
Composed of fat globules, proteins and lactose dissolved in water. Fat globules are large and naturally rise to the top causing cream separation under gravity.
Homogenized milk:
Fat globules are made smaller. Making the fat globules more dispersed, reducing the tendency to rise, thereby improbing creaming stability.
Creaming milk & homogenized milk
Milk: Larger fat globules have a higher tendency to coalesce and rise due to buoyancy.
Homogenized milk: Has reduced creaming due to smaller fat globules and more stable emulsification.
pH stability milk & homogenized milk
Milk: pH can have a large effect on the stability. A drop in pH can lead to protein denaturation, and destabilization of the emulsion, resulting in sedimentaiton
Homogenized milk: The process of homogenization can stabilize the pH more than in milk.
Color milk & homogenized milk
Milk: Color is influenced by the fat content and colloidal dimension of particles such as casein. Larger fat globules scatter light differently compared to smaller ones
Homogenized milk: Appears whiter and more uniform in color due to the uniform distribution of smaller fat globules that scatter light more consistently.
Browian motion and creaming stability
Smaller fat globules in homogenized milk experience higher levels of Brownian motion, which helps keep them suspended by counteracting gravitational forces. In unhomogenized milk, larger globules have reduced Brownian motion, allowing them to coalesce and rise.
Viscosity milk & homogenized milk
Milk: Viscosity is higher due to larger fat globules, as the size increases, the resistance to flow increases.
Homogenized milk: Smaller globule size reduces the viscosity because smaller particles create less resistance.
Newton’s 2nd law and creaming
According to Newton’s 2nd law (F=ma), the force acting on the fat globules due to gravity (weight) and the viscous drag affects their motion.
In larger globules, the gravitational force overcomes the drag more easily, causing them to rise, while in homogenized milk, the smaller globules are influenced more evenly by the viscous forces.
Sol in phases + example
Dispersed phase: solid particles (smaller than one micrometer)
Continuous phase: Liquid
Example: gel, starch in water, blood, paint
Hydrosol in phases + example
Dispersed phase: solid particles or droplets
Continous phase: Water
Example: water-based products made from the distillation of fresh flowers, leaves, fruits, and other plant materials
Suspension phases + example
Dispersed phase: solid particles
Continous phase: Liquid
Example: Mixture of flour and water. Mixture of chalk and water. Muddy water
Emulsion phases + example
Dispersed phase: liquid droplets
Dispersing phase: Another liquid (usually water or oil)
Example: mayonaise, butter, milk
Foam phases
Dispersed phase: Gas bubbles
Continuous phase: Liquid
Aerosol phases + examples
Dispersed phase: Solid or liquid particles
Continuous phase: Gas (usually air)
Example Sea spray, mineral dust, smoke
Retrieve the meaning of viscosity in terms of molecular interactions
Molecular size and shape: larger and more complex molecules can create greater resistance to flow due to their increased surface area and interactions
Intermolecular forces: The strength and type of intermolecular forces significantly impacts viscosity.
Temperature: Higher temperature, is more kinetic energy, decrease in viscosity
Concentration: Higher concentrations can lead to increased viscosity due to more molecular interactions
Flow behavior: Non-newtonian fluids exhibit different viscosities depending on the flow conditions.
Analyse in what respect the results of whipping milk and cream differ from one another in terms of the colloidal characteristics of the two products.
Typically contains about 3-4% fat. This lower fat content limits the amount of fat globules available for stabilizing the air incorporated during whipping.
The low fat content results in a relatively weak foam that is less stable. The air bubbles tend to coalesce, leading to a quicker breakdown of the foam. Milk can only be whipped to a very limited degree and is not suitable for applications requiring a stiff or stable foam.
In milk, the smaller and fewer fat globules contribute less to foam stability. The air bubbles lack sufficient anchorage, leading to instability.
Emulsifiers are less concentrated, reducing the capacity to stabilize the foam formed during whipping
Analyse in what respect the results of whipping milk and cream differ from one another in terms of the colloidal characteristics of the two products.
Contains around 30-40% fat or more. The higher fat content provides a greater quantity of fat globules necessary to trap air and form stable bubbles.
Has a higher concentration of proteins, particularly caseins, which enhance the stability of the foam during whipping. The presence of fat globules also supports the protein network that forms.
The high-fat content allows for the creation of a much lighter and more stable foam. When whipped, the fat globules partially coalesce and form a network that holds air bubbles, producing a thick, stable whipped cream. The foam can maintain its structure for an extended period and can be used in various culinary applications.
In cream, the larger and more numerous fat globules create a rich network that supports the air bubbles during whipping.
on the other hand, benefits from a higher concentration of emulsifiers, which promotes better foaming and stabilization of air pockets.
Viscosity Formula for Concentric Cylinder
n = (F x h)/((2pi x r2) x w)
n= viscosity (Pa) (mPa per s to Pa per s you divide by 1000)
F = force (N)
h = height of cylinder (m)
w = angular velocity (rotation speed) (m)
r = radius (m)
How small are fat globules in homogenous milk?
Smaller than one micrometer
Properties of casein micelles
Proteins
Exhibit Brownian motion and therefore do not clump
When pH is lower than 5.3 only then they start to clump
Formula upward force of a particle in a fluid
F = (4/3) x (π) x (g) x (△p) x (R3)
g=gravitational acceleration
△p = The difference in density of the two fluids
R = radius of the particle
10 N = 1 Kg
Formula quantifying thickness of a fluid
F = n x v x A/D
F = Force
N= viscosity (Pa)
A = Surface area A (m)
D= Height (m)
V= velocity
Formula creaming velocity
U = (2a2 x Δp x g)/9 x n
a = radius (m)
Δp = Density difference (Kg/m3)
g = Gravitational acceleration (10 m/s2)
n= viscosity = Pa
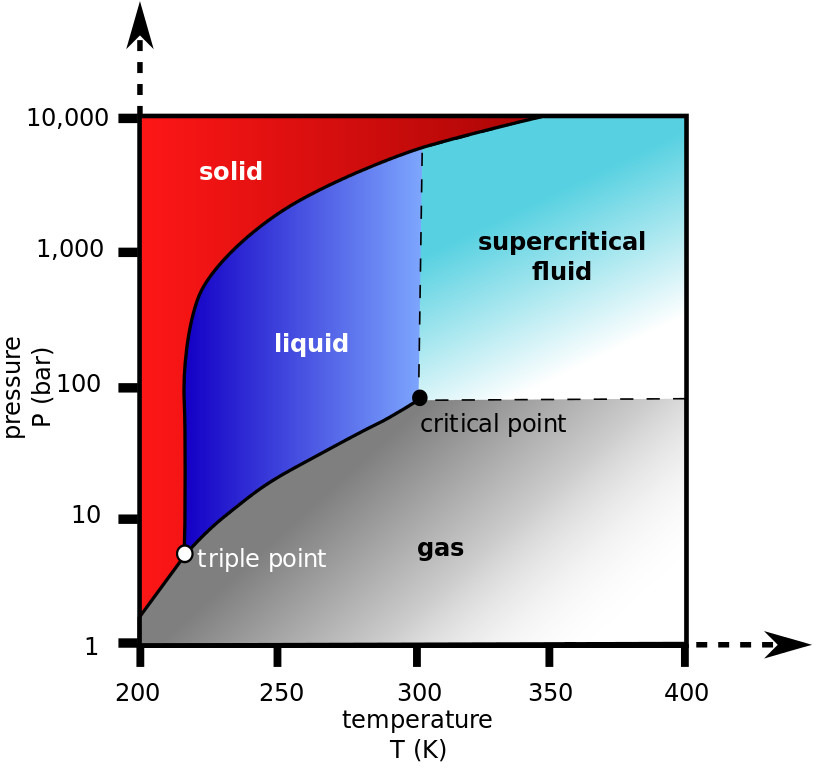
States of matter in a graph pressure vs. time