Chemical reactivity -acids and bases
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms


Weak base (molecular)
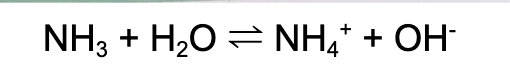


Strong acid
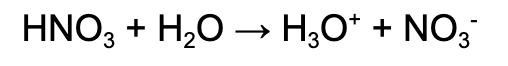


Strong base
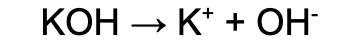


Strong acid
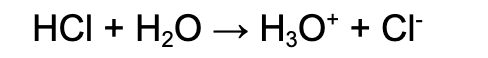
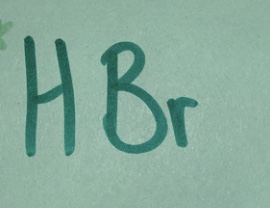
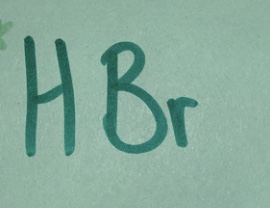
Strong acid
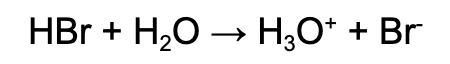
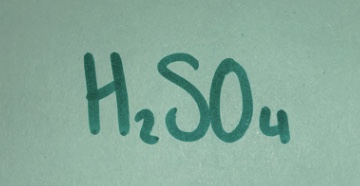
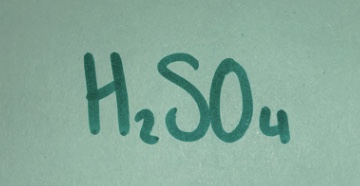
Strong acid


Strong base



Weak acid
(non neutral salt)
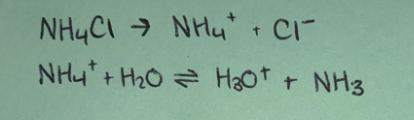
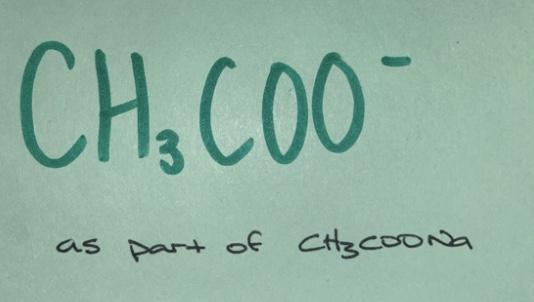
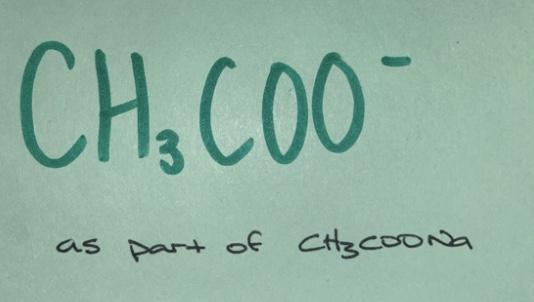
Weak base
(non-neutral salt)
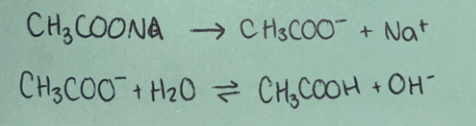
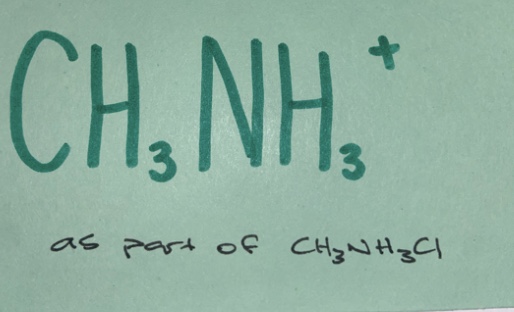

Weak acid
(non-neutral salt)
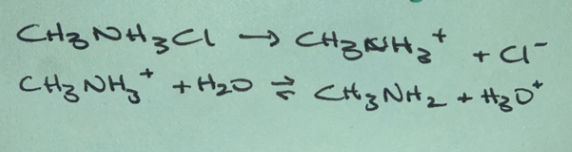
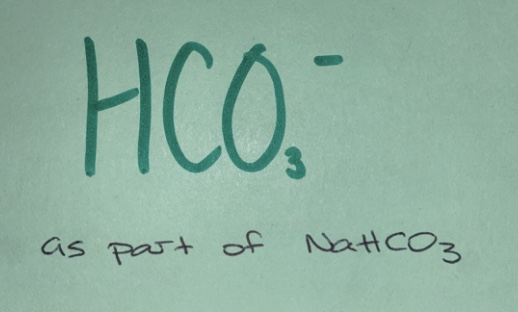
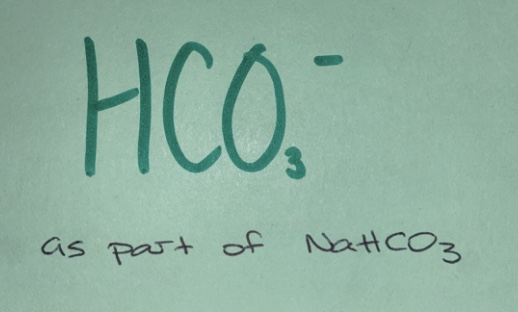
Weak base
(non-neutral salt)
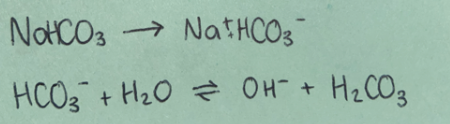
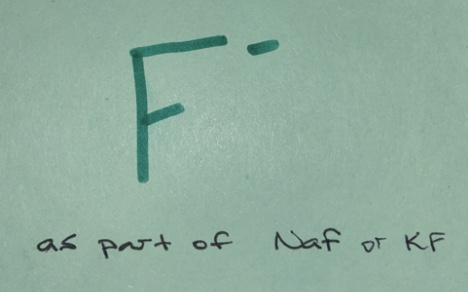
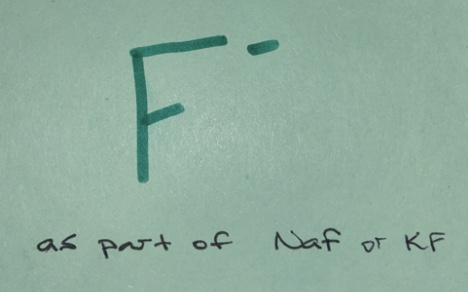
Weak base
(non-neutral salt)
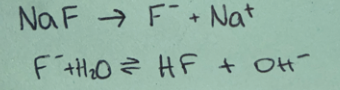
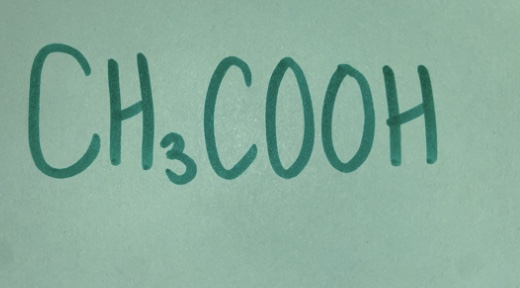
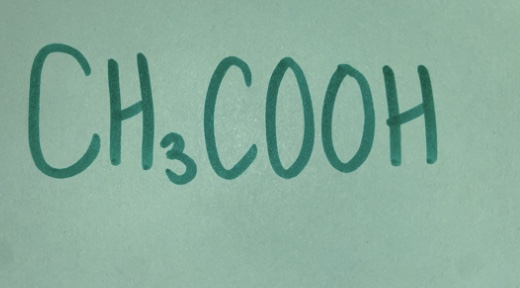
Weak acid
(mollecular)
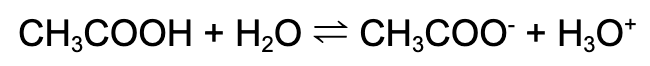


Weak acid
(molecular)
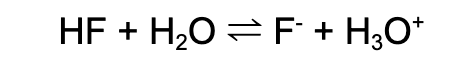
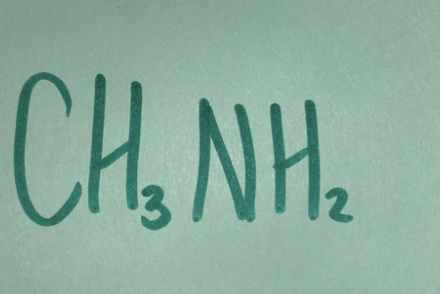
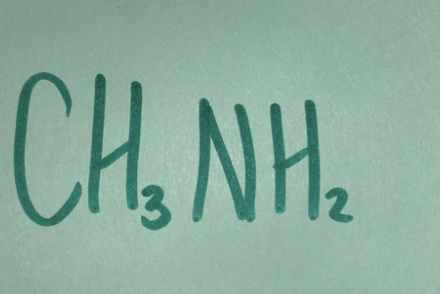
Weak base
(mollecular)
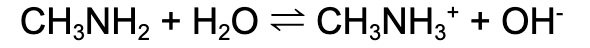
Conductivity
Str. Acid
Str. base
Salt
Fully dissociates
High [ions]
Good conductors
Strong acid
Fully dissociates in H20 >
(full arrow, not equation)
Acids
Proton donor
(increases (H30+) in water)
Conductivity
wk acid
wk base
Portial dissoc
Low (ions)
Poor Conductors
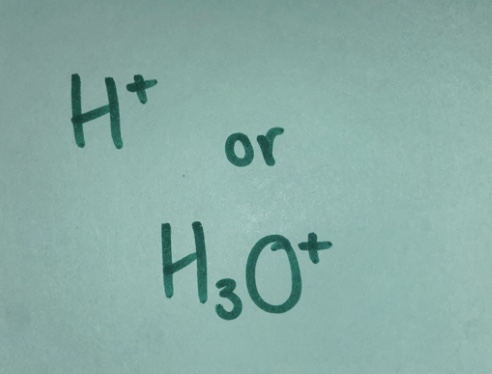
Proton
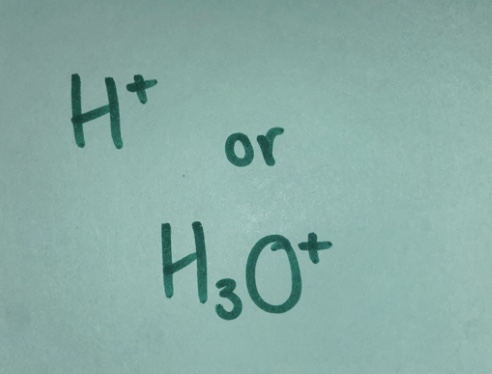
Conductivity depends on
(ions)
pH
Str base
Full dissoc
High (OH-)
Low(H30+)
High pH
Amphiprotic
a species that can donate or accept a proton
eg. H20, HCO3-
Weak(acid)

Bases
Proton acceptor
(increases (OH-) in water)
pH
Str acid
Full dissoc
High(H3O+)
Low pH
R.O.R
wk acid
Full dissoc
High(H3O)
Lots of particles/mL
Lots of collisons/s
Fast R.O.R
R.O.R
wk acid
Partial dissoc
Low(H3O+)
Fewer particles/mL
Fewer collisons/s
Slower ROR
pH
wk acid
Partial dissoc
Lower(H3O+)
Higher pH
Conjugate acid/base pairs
Species that differ by 1 x H+
Do we need to include water in the equation
Not if its a salt nor a strong base