ZOOL 461 Midterm 1
1/71
Earn XP
Description and Tags
ZOOL 461 UofC
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
LECTURE 1: Introduction & Mechanics
Hormones (2)
Chemical messengers produced by one cell to regulate activity of another cell, and delivered by means of endocrine, neuroendocrine, paracrine, autocrine, neurocrine, or pheromonal route
Involved in all aspects of body physiology (including reproduction, growth & development, homeostasis, storage)
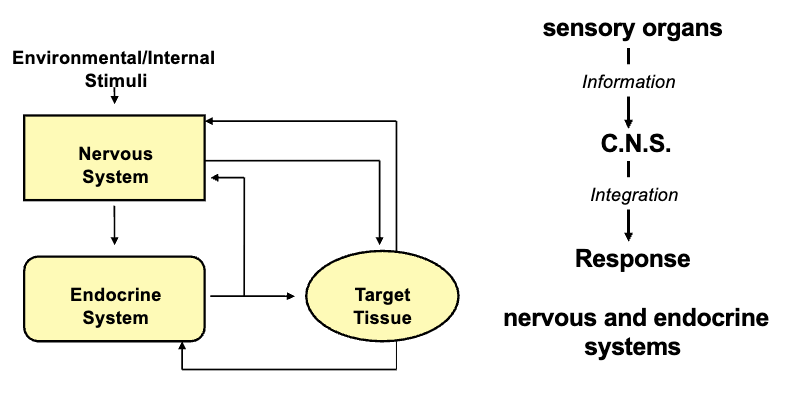
Nervous System & Endocrine Glands Are Interrelated (2)
Any changes that happen are detected by various receptors through the neuronal system that then provide the sensory organs with information
The nervous system controls rapid activities; the endocrine system regulates the slower functions
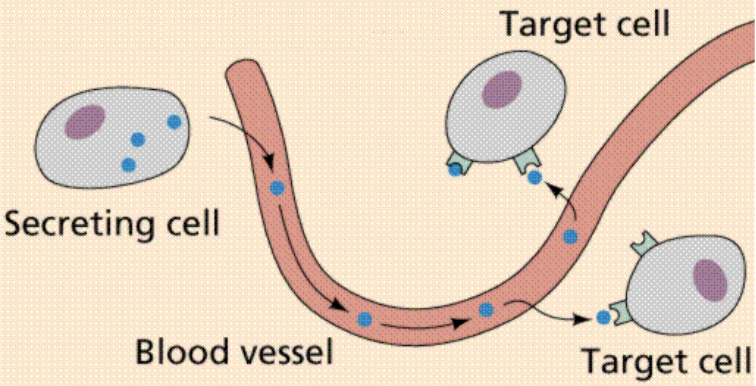
Endocrine
Hormone released by secretory cells, and then enters blood circulation to be transported to the site where target cells are located with specific receptors
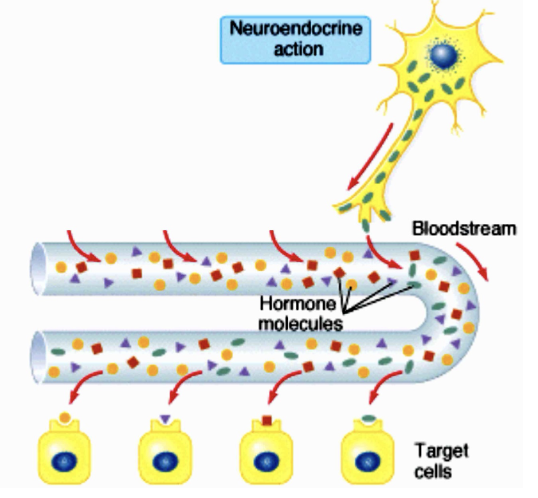
Neuroendocrine
The hormone is released by nerve cells into the circulation and is transported to the target cells
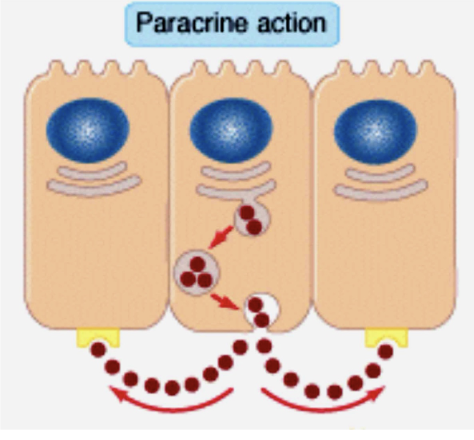
Paracrine (2)
The hormone is released and diffuses to its target cell through the intermediate extracellular fluid
Secretory cells are close to the target cells, so hormones do not need to enter the blood (acts on neighbouring cells)
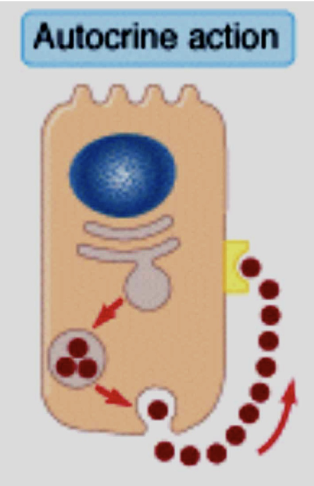
Autocrine
The target of the secreted hormone is the same cell that released it
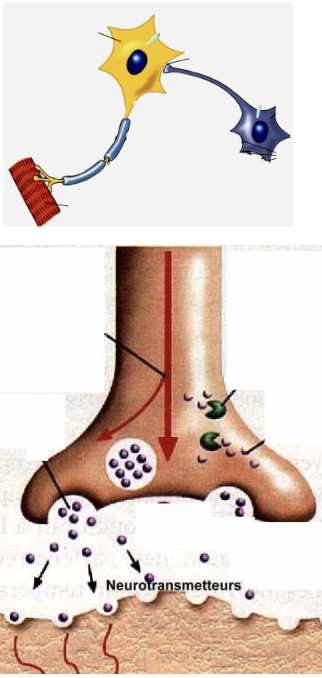
Neurocrine (2)
Neurons secrete the hormone in the immediate vicinity of the target cell
Chemical substance is delivered directly at the synaptic cleft; very localized and produced within that particular cell to act on a receptor
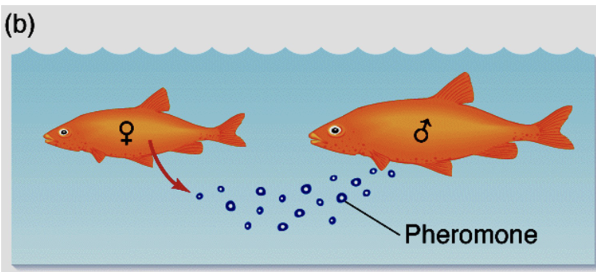
Pheromone (2)
The hormone is released into the environment to induce a biological response in another animal
It is usually species specific and may also be called exocrine action
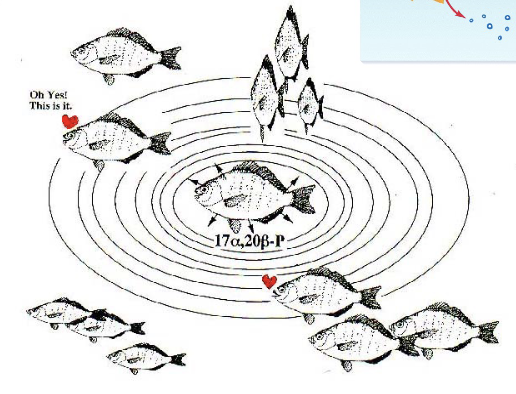
Pheromone: Fish (2)
Some fish use a hormone that is excreted through pee and goes into the ocean for circulation
Very dilute concentration and acts on the nasoreceptors of male fish to let them know females are ovulating
Molecules Involved in Information Transfer Include: (4)
Peptides and proteins
Steroids (derived from cholesterol)
Amino acids and amino acid derivatives
Eicosanoids (hormones that are fat-soluble and associated with membranes)
Receptors
All receptors are glycoproteins (proteins with carbohydrate moieties)
Receptors form elaborate shapes through the function of secondary, tertiary, and quaternary structures
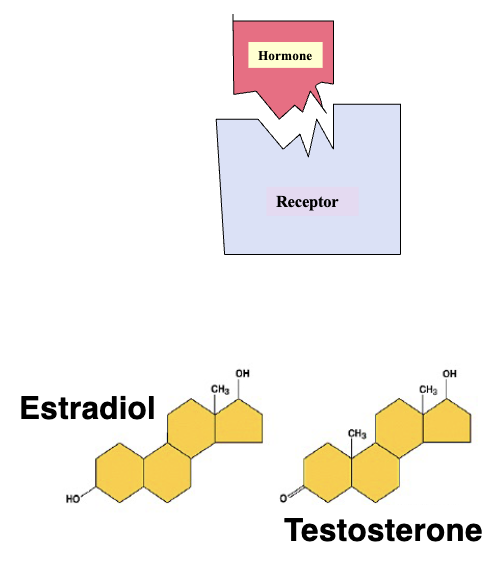
Hormone Receptor Interaction (2)
Hormone interact with their target cells by binding to specific molecules called receptors
Hormone specificity is achieved by a Lock & Key Mechanism
Receptor Activation Requires: (2)
Correct shape
Correct charge
Receptors Have Two Functions: (2)
Recognition: Specific binding
Transduction of signal
Agonists
Molecules that can stimulate biological activities
Antagonists
Blocks biological activity
Competitive Antagonists
Bind but do not stimulate biological activity (competitive inhibitors)
Hormone-Receptor Interaction is ___ and ___
Rapid
Reversible
Hormone Receptor Rate Constants: Association Rate Constant (3)
Association rate constants are the forward reaction defined by when hormones are secreted and begin binding to receptors to form hormone-receptor complexes
Is a function of time
Equation is K+1 and is in units of M-1sec-1

Hormone Receptor Rate Constants: Dissociation Rate Constant (2)
Is the reverse reaction that defines binding affinity and is the measure of how fast the hormones dissociate from receptors once equilibrium is reached
Has units of sec-1

Equilibrium (2)
For every one molecule that associates, one dissociates
Reaching a plateau requires all receptors to be activated as long as sufficient hormones are present
Affinity
Higher affinity of hormone for a receptor means less of that same hormone needed to activate the receptor (increased potency)
Hormone Receptor Rate Constants: Equilibrium Association Constant (Ka) - Affinity (3)
Defined as Ka which is the ratio of K+1 to K-1
Measured in units of M-1
Ka is no longer a function of time, but instead affinity

Kd (2)
Reciprocal of Ka is Kd; calculated as it is a parameter analogous to determining potency
Measured in units of M

Ka vs. K+1
One happens at equilibrium while one happens as a function of time; once equilibrium is reached then we have Ka
Dose Response Curve (2)
Concentration of hormone vs. biological response generates a sigmoidal relationship
Increasing dose of hormone increases responsiveness until all receptors are occupied (plateau)
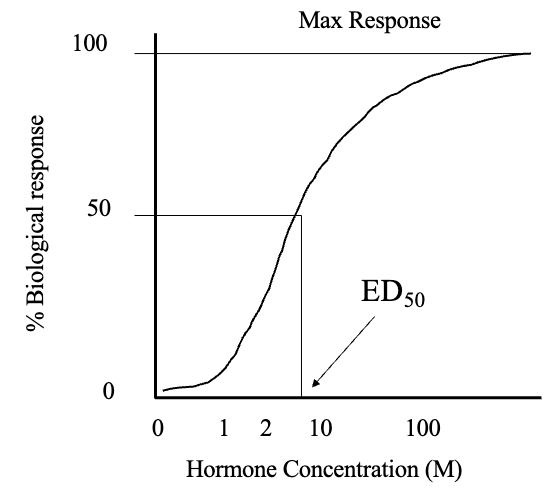
ED50 (3)
Is the effective dose giving half maximal response
This is a determination of hormone potency and is analogous to Kd, as by estimating potency of hormone you can estimate Kd and indirectly calculate Ka
Has units of M (mol/L)
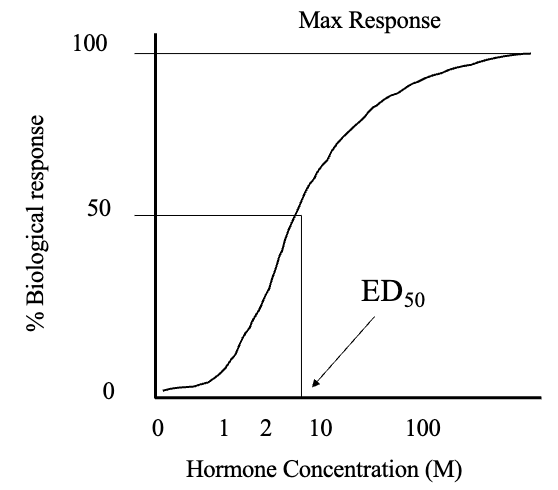
Receptor Regulation (3)
Both receptor binding affinity (Ka) and capacity are regulated
Capacity is the number of receptors
Receptor capacity is regulated by various functions, but affinity does not change without anything pathological that changes the nature of the receptor
Upregulation
Increased receptor synthesis and availability
Downregulation
Decreased receptor synthesis and availability
Receptor Regulation on Dose Response Curve: General Trends (2)
Changes in receptor capacity affect maximum responsiveness
Change in receptor affinity changes ED50
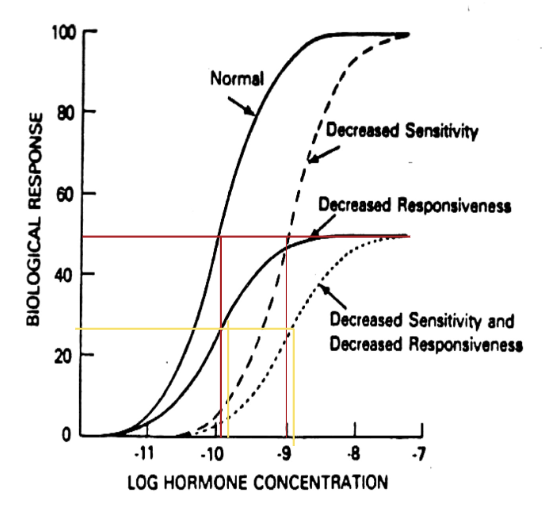
Receptor Regulation on Dose Response Curve: 3 Lines
Decreased affinity creates dashed line
ED50 is greater than normal, means less potency; need more of the same hormone to elicit same physiological response
Decreased responsiveness creates solid line
Downregulation of receptor number due to degradation creates a new response curve with decreased responsiveness, but same ED50
Decreased sensitivity and responsiveness creates dotted line
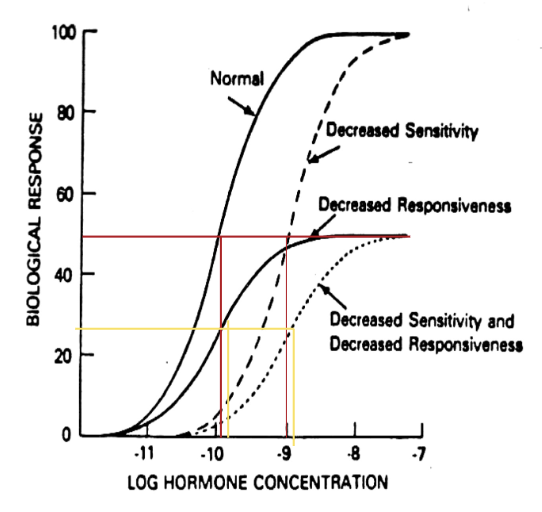
Hormone Receptors Fall Into Two Categories: (2)
Intracellular Receptors
Plasma Membrane Receptors
Intracellular Receptor (3)
Receptor with binding domain is inside the cell
Hormones must first penetrate the membrane and find the receptor to act on the cells
Examples are Iodothyronines (thyroid hormones) and steroids
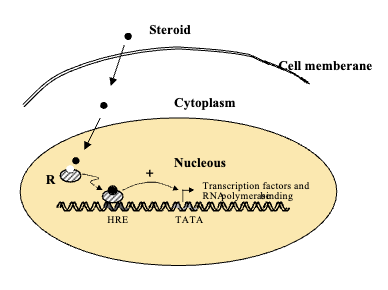
Plasma Membrane Receptors (2)
Binding domain faces outside the membrane and lies at the cell surface (hormones do not need to penetrate)
Examples are peptide, protein, and catecholamines
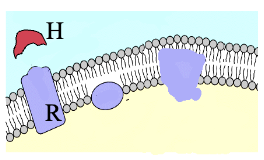
Exception: Plasma Membrane Receptors
Few cases where receptors are on the outside of organelles inside the cells instead of cell surface, therefore hormone produced inside the cell and acts on those organelles
Membrane Receptors Exist Within The Lipid Bilayer (3)
At physiological temperature, receptors in the bilayer are mobile due to cholesterol
Cold = less fluid; molecules not functioning as well (hypothermia)
The relevance of this fluidity is that molecules can move laterally to allow for interactions between receptors, G-proteins, and receptor aggregation (dimers)
Initiation of Biological Response By a Hormone Involves: (2)
Specific binding
Transduction of signal coupled to intracellular effectors system
Intracellular Receptors: Steroid vs. Thyroid Hormones (2)
Steroids are hydrophobic and can easily pass through the membrane (diffusion)
Thyroid hormones are not hydrophobic and require transporter molecules that facilitate the movement into the cell (facilitated diffusion)
Binding Proteins (2)
The half-life of hormones is increased by binding to binding proteins which can be specific or non-specific
Binding proteins allow hormones to last longer in circulation before going to their target tissue
Intracellular Receptors: Cytoplasm vs. Nucleus
Sometimes receptors are in cytoplasm and hormone binds to cytoplasmic receptor and then moves into nucleus to interact with HRE
Sometimes receptors are inside the nucleus meaning the hormone penetrates the nucleus and binds receptor to hormone response element (HRE) on DNA
Intracellular Receptors Are Also Known As ___
Ligand-activated transcription factors
Each Intracellular Receptor Contains: (3)
Hormone Binding Domain
DNA Binding Domain
Activation Domain
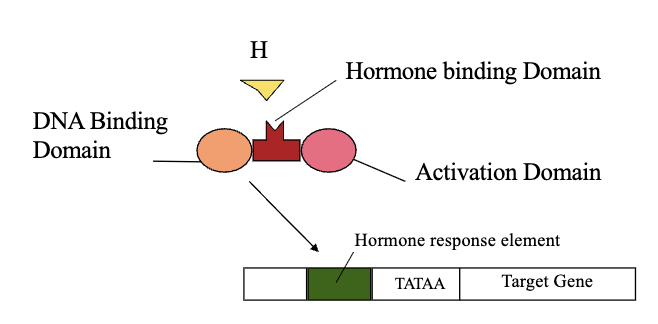
Mechanism of Ligand-Activated Transcription Factors
The activated hormone-receptor complex interacts with a specific sequence of DNA referred to as a hormone response element (HRE)
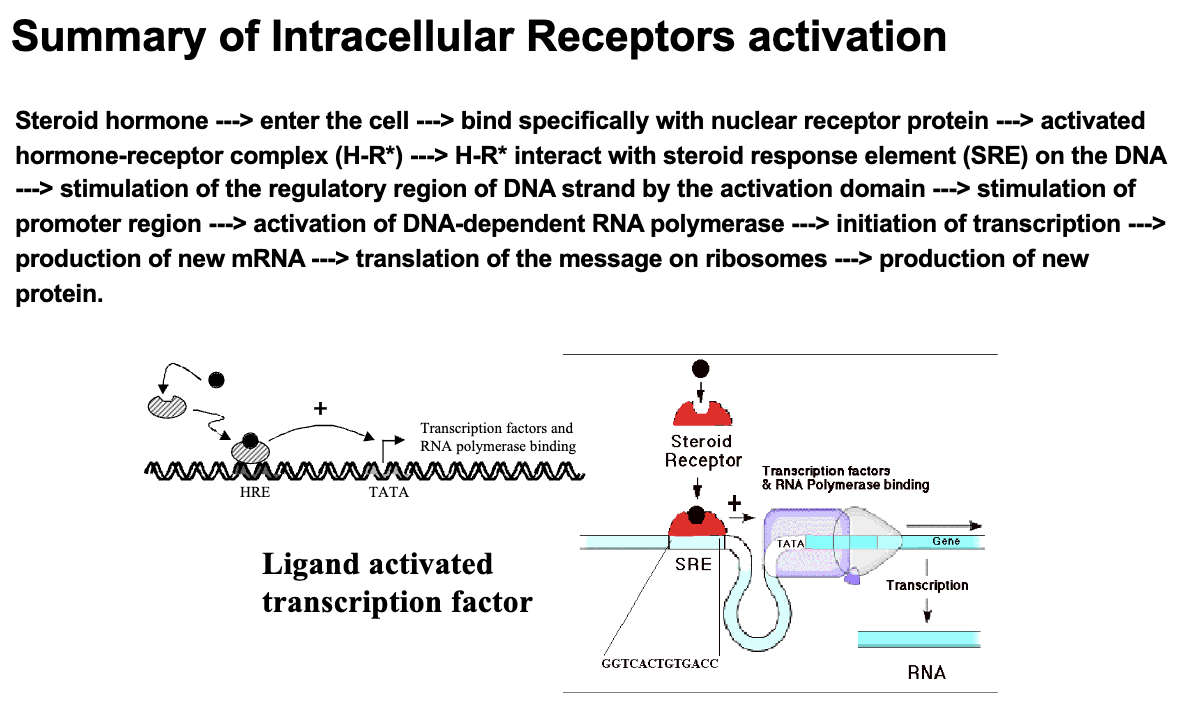
Steroids & Thyroid Hormones Act With Both ___ and ___
Intracellular Receptors
Membrane-Associated Receptors
*In MC question; they only act with intracellular receptors, but in short answer both can be explained
The Receptor-Effector Coupling Can Be Achieved By: (2)
A process intrinsic to the receptor (same molecule)
Through interaction of the receptor with other membrane proteins (two separate molecules coupled by a tertiary molecule)
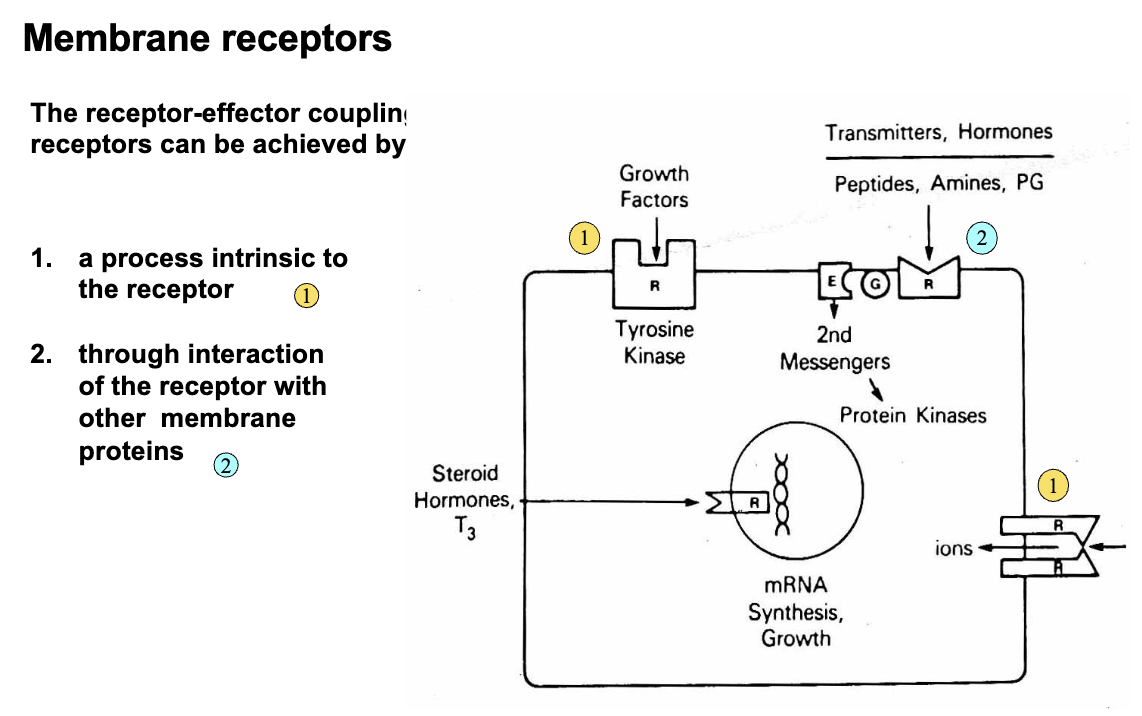
The Effector Can Be An ___ or ____
Enzyme
Ion Channel
Intrinsic Receptor: Effector Is An Enzyme - 3 Examples
Tyrosine Kinase: Enzyme that typically phosphorylates either serine or threonine residues
EGF
Insuline
Tyrosine Kinase Receptors (RTKs) Consist of: (3)
A single protein chain with extra cellular domain that binds the hormone
A single transmembrane region of 20-22 amino acids
An intracellular domain that has a tyrosine kinase catalytic domain
RTKs: EGF Receptor (4)
A single protein chain that has different domains
Extracellular domain which contains the hormone binding domain that binds to the receptor
Transmembrane domain which consists of hydrophobic amino acids and has leucine residues which form an α-helical structure
Cytoplasmic domain has the enzyme part with tyrosine kinase activity that phosphorylates residues in target molecule
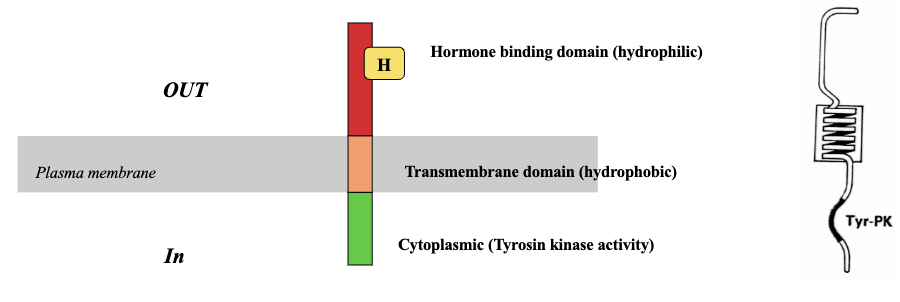
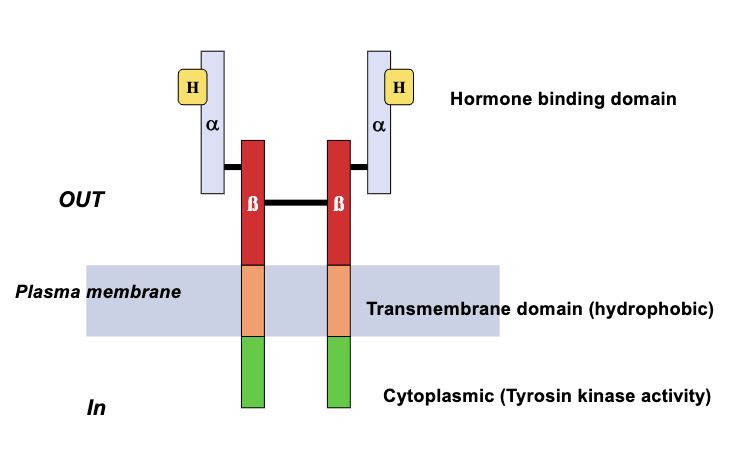
RTKs: Insulin & IGF-1 Receptors (4)
Two subunits: α and β
β subunit has extracellular, transmembrane, and intracellular domains
α subunit is extracellular and attached covalently to β subunit and contains the hormone recognition site
Once activated, the two subunit molecules move laterally to dimerize and phosphorylate at the tyrosine residue
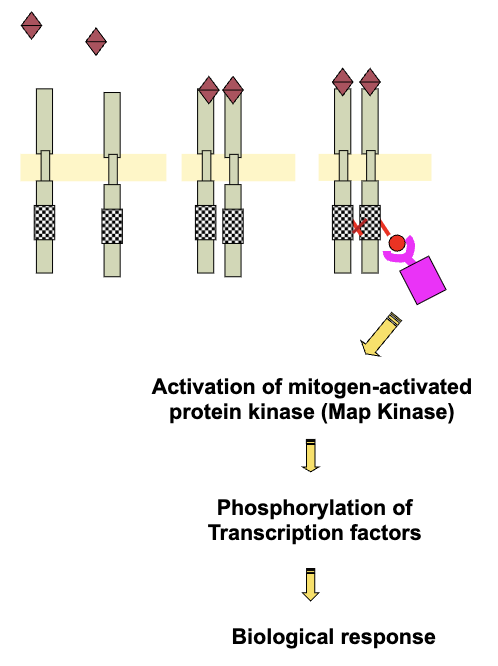
Pathway For Activation of RTK With Intrinsic Kinase Activity (4)
Hormone binding to extracellular domain
Dimerization
Activation of tyrosine kinase in the intracellular domain
Autophosphorylation/trans-phosphorylation of the receptor
RTKs & Phosphorylation (3)
Adaptor proteins getting phosphorylated activates other molecules (e.g. mitogen-activated kinases - map kinases)
Map kinases are enzymes that get activated and further phosphorylate certain transcription factors which can then cause transcription leading to biological responses
Difference with ligand-activated is they do not need to bind to hormones (once phosphorylated are activated until they degrade)
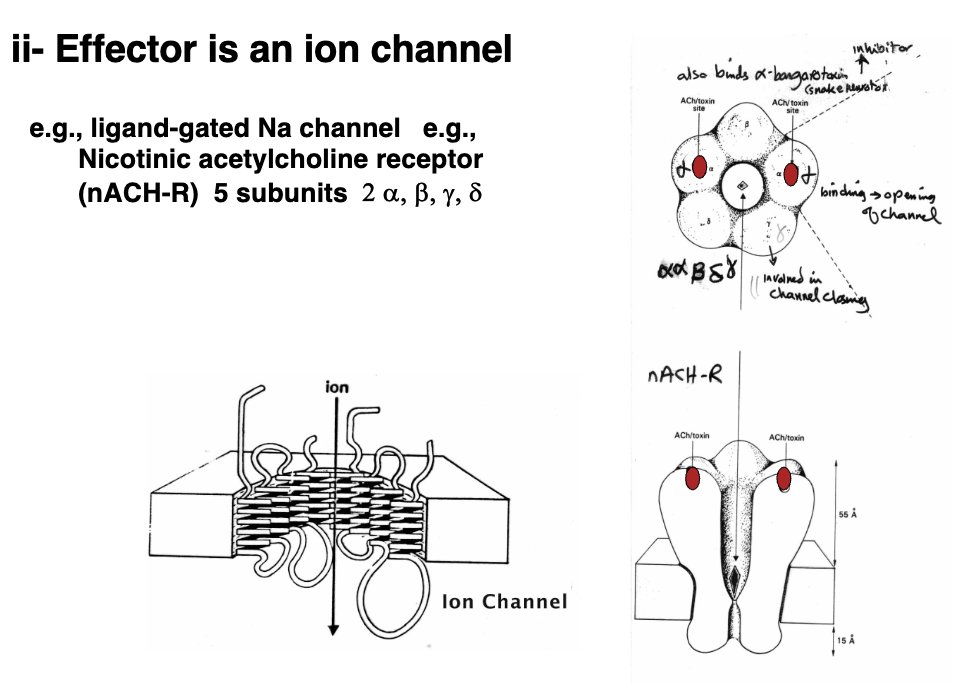
Intrinsic Receptor: Effector Is An Ion Channel - Acetylcholine Receptor (2)
Example would be a ligand-gated Na channel like the nicotinic acetylcholine receptor
Binding domain for the acetylcholine receptor is located on the α-subunit, and once acetylcholine is released it activates the binding domain and opens it up so sodium can pass through
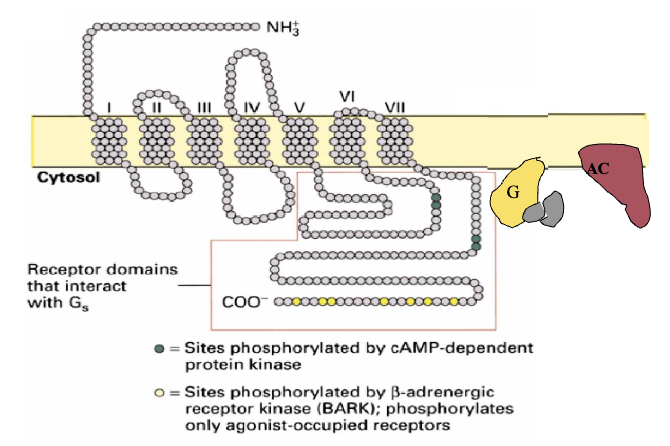
Coupled Receptor & Effector: G-Protein Coupled Receptors (4)
Belongs to a family of receptors that consist of the receptor, effector, and G-protein
Characterized by having 7 transmembrane domains; loops which are either extracellular and/or intracellular
Specificity for G-protein brought about by sequence of amino acids and the way it dictates conformation
Ligand binds to hormone-binding domain leading to a conformation change within the receptor to form an affinity and activate G-protein
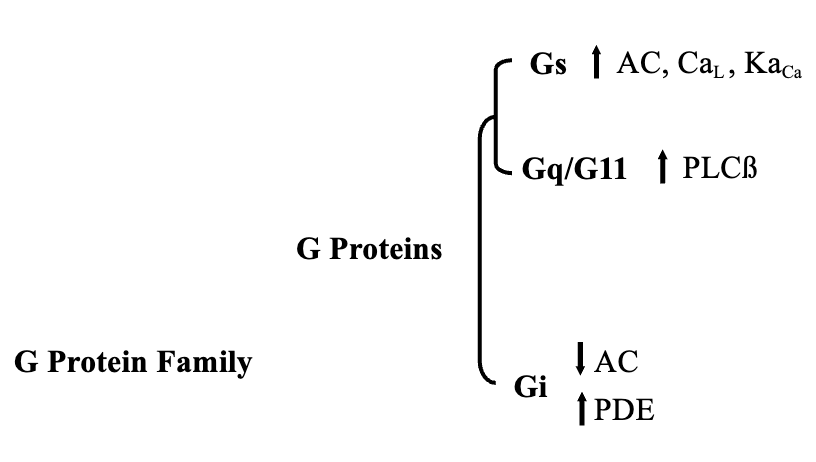
3 Different Types of G-Proteins
Stimulatory G-Protein (Gs)
Activates adenylate cyclase
Inhibitory G-Protein (Gi)
Inhibits adenylate cyclase
Activate Phospholipase Cβ (Gq/11)
Guanine Nucleotide Regulatory Protein: G-Protein (3)
G-protein works as a heterotrimer (3 subunits); consists of α, β, γ
Guanylate diphosphate on α subunit is associated with the inactive form of G-protein (GDP) which means it has an affinity for β and γ subunits
Once receptor is activated and associates with inactive G-protein, first thing that happens is GDP gets swapped with GTP causing it to lose affinity for β and γ subunits, and split into the active form of G-protein with α-subunit
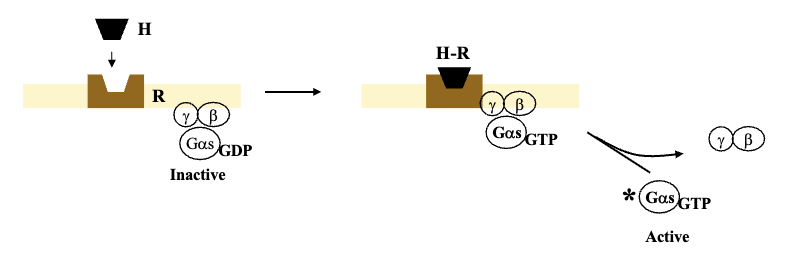
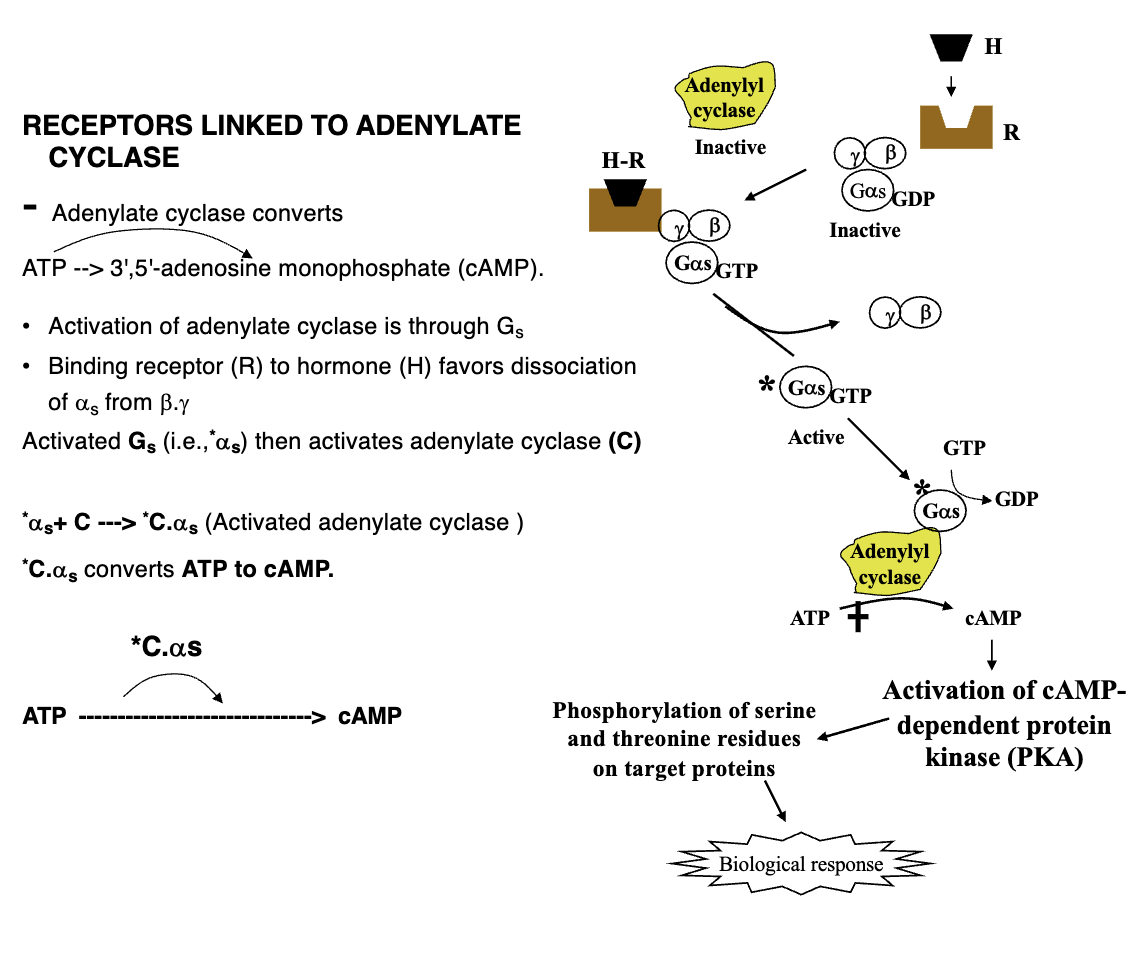
Adenylate Cyclase Activation Pathway (6)
Activation of adenylate cyclase through Gs
Binding receptor to hormone favours dissociation of αs from β, γ
Activated Gs (*αs) then activates adenylate cyclase (C); simultaneously G-protein dephosphorylates GTP into GDP causing it to become inactive by forming affinity for β, γ subunits
Adenylate cyclase then converts ATP to cAMP
Activation of cAMP-dependent protein kinase (PKA)
Phosphorylation of serine and threonine residues on target proteins leading to biological response
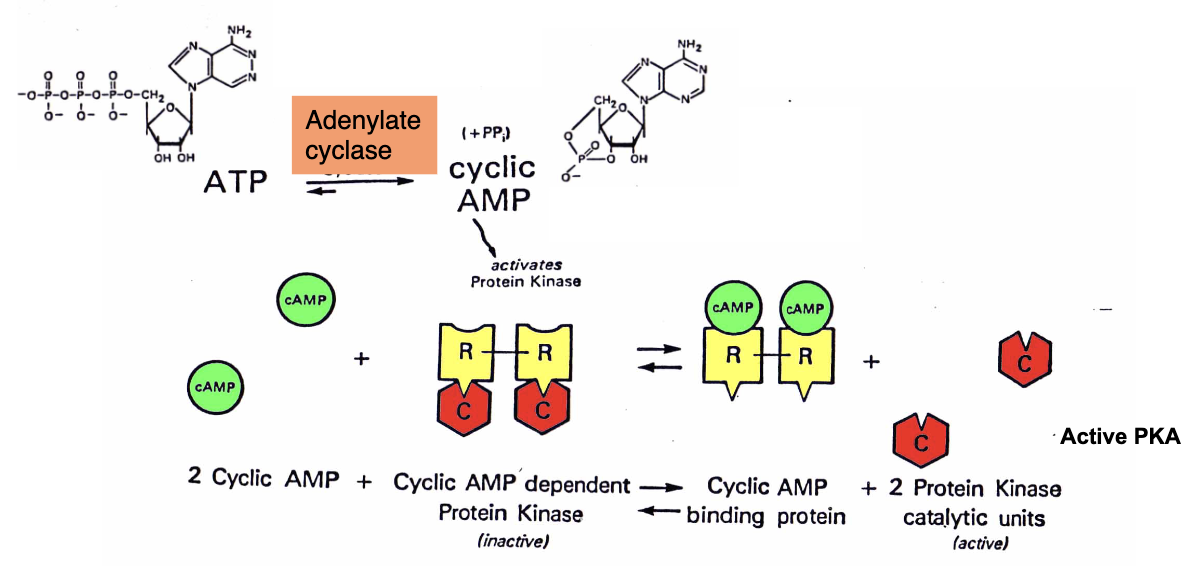
cAMP-Dependent Protein Kinases (3)
PKA exists in an inactive form made of two regulatory subunits (R) and two catalytic subunits (C)
cAMP binds to the regulatory subunits causing them to dissociate
The active catalytic subunits then phosphorylate serine or threonine residues on target proteins
Reversible Action of Phosphoprotein Phosphatases on cAMP (3)
Phosphodiesterase breaks down cAMP into AMP, and the breaking of the cyclic bond causes it to become inactive
Without cAMP, PKA regulatory subunits rebind the catalytic subunits thus inactivating PKA
With cAMP gone, phosphoprotein phosphatases come in and remove the phosphate groups added by PKA by hydrolysis, restoring proteins to baseline
Phosphodiesterase (3)
Inactivates cAMP by hydrolyzing the cyclic ring to 5’ AMP
Phosphodiesterase is inhibited by family of methylxanthines (e.g. caffeine)
Phosphodiesterases also inactivate cGMP
cGMP (3)
cGMP is responsible for relaxation of smooth muscle
Phosphodiesterase type 5 (PDE 5) is responsible for cGMP hydrolysis in smooth muscle
PDE 5 thus allows smooth muscles to contract again
PDE 5 & Viagra (Sildenafil Citrate)
Drugs like Viagra inhibit PDE5, so cGMP sticks around longer which means prolonged smooth muscle relaxation (increased blood flow in erectile tissue)
Inhibitory Actions of Hormones on Adenylate Cyclase Activity (3)
Receptors coupled to Gi inhibit adenylate cyclase activity (e.g. opioids)
The regulatory component is α1, and this process turns off the active enzyme and prevents the conversion of ATP to cAMP
Gi also stimulates phosphodiesterase which breaks down existing cAMP and cGMP
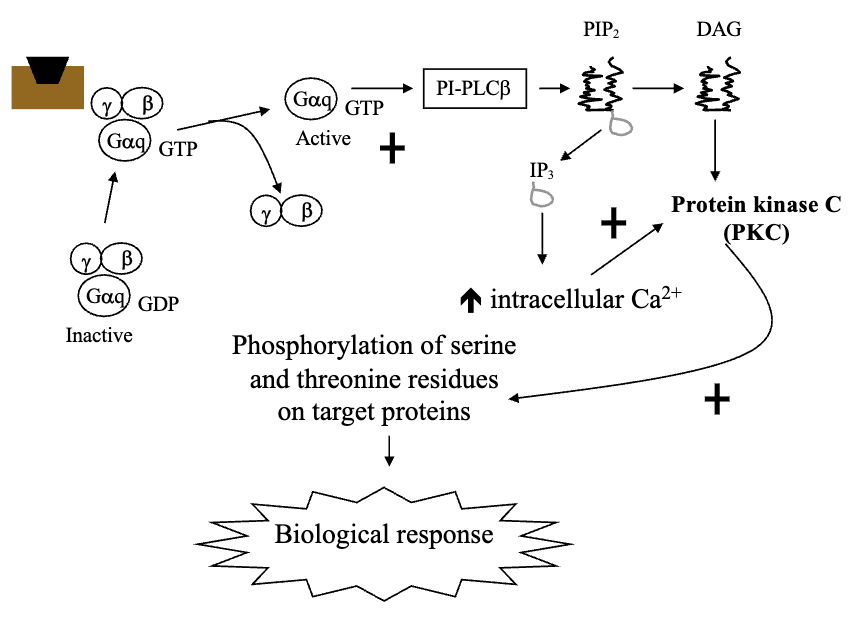
Hormone Receptors Linked to Phosphatidylinositol Turnover Via Gq/G11 Protein (4)
Calcium-mobilizing hormones elicit cellular responses by activating phosphatidylinositol turnover by G-protein dependent mechanisms (Gq)
Gq activates Phospholipase-Cβ
PLCβ converts phosphatidylinositol 4,5-biphosphate to two intracellular messengers: Diacylglycerol and Inositol Triphosphate (IP3)
Diacylglycerol activates protein kinase C, while IP3 releases Ca2+ from non-mitochondrial intracellular stores (primarily endoplasmic reticulum) and increases cytoplasm Ca2+
IP3 & Endoplasmic Reticulum (2)
Endoplasmic reticulum has ligand-gated calcium channel which is activated by IP3
Calcium rushes out after binding as it goes from hyperosmotic to hypoosmotic environment through electrochemical gradient
Physiological Calcium (3)
Concentration of free calcium in cytoplasm is 0 as it is quickly sequestered into the endoplasmic reticulum and mitochondria
Calcium has +2 charge meaning release into cytoplasm would increase charge inside the membrane relative to outside (changing polarization)
Activation of PKC is calcium-dependent, and it not only changes polarity but also increases PKC to phosphorylate other proteins
Calcium in Endoplasmic Reticulum vs. Mitochondria (2)
ER: Calcium is labile and can be mobilized
Mitochondria: Calcium cannot be mobilized (calcium-dependent functions)
Calcium-Mediated Hormonal Response
Ca2+ plays a major role in regulation of cellular activity which includes: phosphodiesterase, calcium pumps, adenylate cyclase, and phosphorylase kinase
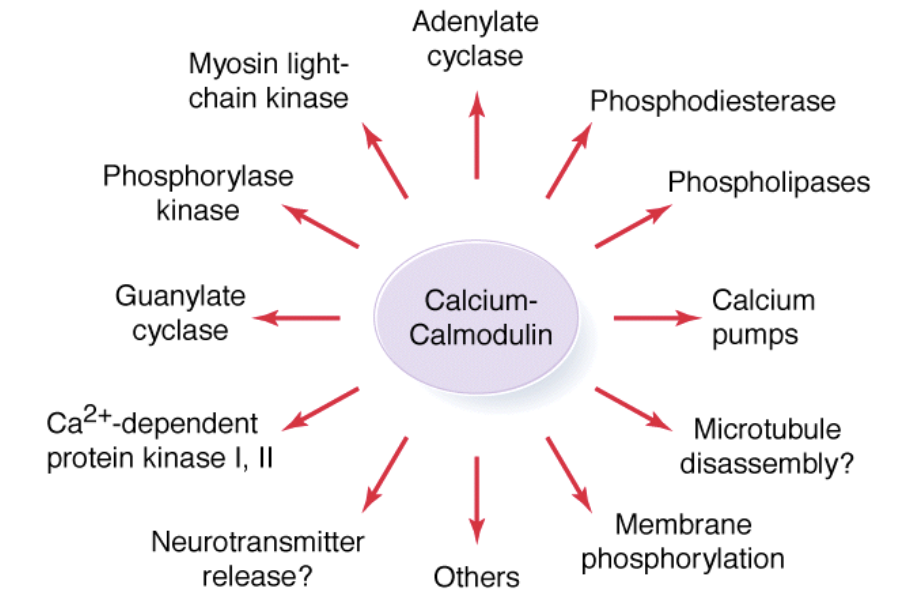
Calcium Gets Sequestered Quickly Because ___
Calcium itself activates calcium pumps which pump it into compartments
GPCR Coupled to Ion Channels