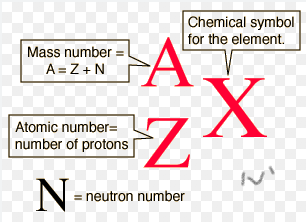Polar and Non polar Molecules, VSPER Notation
0.0(0)
Card Sorting
1/8
Last updated 10:57 PM on 3/1/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
1
New cards
What are intermolecular forces? (IAF)
The forces of attraction between molecules
2
New cards
What are the 3 intermolecular forces? (IAF, hint: DDH)
Dispersion forces, Dipole interactions, and Hydrogen bonds.
3
New cards
What are dispersion forces and which molecules have them?
Forces that are created from random movement of electrons, causing a temporary dipole. all molecules have them
4
New cards
What are dipole interactions, (HINT: magnet) and what molecules have them?
\+ end attracted to the - end, only to POLAR molecules
5
New cards
What are Hydrogen bonds, and which molecules have them?
a hydrogen bond is when the hydrogen molecule is bonding with a lone pair, usually in molecules with nitrogen, oxygen, or fluorine. They also have HIGH electronegativity.
6
New cards
What is a bond?
atoms combinding
7
New cards
what is an ionic bond?
the electrostatic force that holds ions together in an ionic compound.
8
New cards
Which of the following determines the geometry around a central atom in a Lewis structure?
The number of valence-shell electron pairs
9
New cards
what is the format for isotopes?
a/z X, (A= Z + N) (Z=+) (X= chemical symbol)