core practicals
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
microscopy practical aim
investigate biological specimens using microscopes
microscopy practical safety points
safety goggles when handling iodine
broken glass risk
microscopy practical method
peel off an onion layer (forceps)
place onto slide with a drop of water (pipette)
two drops of iodine solution
place cover slip
remove excess stain (paper towel)
place the slide on the stage
microscope focus: start low, focus, then move up
make a labelled drawing
enzyme and pH practical aim
calculate the rate of enzyme activity at different pH values
enzyme and pH practical safety points
avoid contact with iodine → irritant
be careful with hot water
enzyme and pH practical controlled variables
temp
volume and concentration of starch or amylase solutions
time interval between testing
enzymes and pH practical method
on a tile, label each tile with time
add one drop of iodine each
starch, amylase, buffer solutions in water bath at 25 degrees for a few minutes
add 2cm³ of buffer solution (and mark pH) and amylase and starch to the test tube
start timing
put a drop of the mixture onto each well every thirty seconds until iodine remains brown (instead of turning black)
repeat steps 1 - 6 and take a mean time
calculate rate of enzyme reaction → 1/time taken
repeat steps 1 - 8 with buffer solutions of different pHs
plot a graph for the rate of reaction against pH
test for starch method
some food sample into test tube
add a few drops of iodine
iodine test for starch positive result
brown → blue-black
benedict’s test aim
test for reducing sugars
benedict’s test method
add an equal volume of benedict’s solution to the food sample
place in a hot water bath
benedict’s test positive result
red (present) → blue (no sugar present)
test for protein method
add a few drops of Biuret’s reagent to the food sample
shake
test for protein positive result
blue → purple
test for lipids method
a few cm³ of ethanol to the food sample
add equal volumes of distilled water
test for lipids positive result
a white emulsion forms on the surface
emulsion test aim
test for lipids
food tests safety precautions
tie hair back
wear safety goggles
biuret solution may be corrosive
ethanol is flammable
osmosis practical aim
immerse plant tissue in a range of concentrations to investigate osmosis
osmosis method
cut potato cylinders (cork borer)
trim them to 3 cm
measure and record the mass
10cm³ of 1.0 sugar solution into a boiling tube and label
repeat step 4 for the other concentrations
add one potato cylinder to each boiling tube
leave for 15 minutes
remove and blot dry
measure the change in mass
calculate percentage change
plot change in mass against conc (x-intercept is the concentration of sugar that is isotonic to the potato)
osmosis controlled variables
temperature
type of potato
age of potato
osmosis source of error
different parts of the potato may have different water potentials
osmosis risk
cork borer and sharp knife
microbiology practical aim
investigate the effect of different antiseptics or antibiotics on bacterial growth
microbiology method
disinfect
mark the agar plate with 3 segments with dots in the middle, initials and date
wash your hands
add the bacteria with a sterilised inoculation loop
place the different antiseptics onto different filter paper discs
place each filter paper onto the dots and note the antiseptic
tape the lid loosely
incubate at 25 degrees for 48 hours
measure the diameter of the clear zones (do twice and take a mean)
calculate zone of inhibition → (pi)r²
microbiology controlled variables
species of bacteria
area of filter paper disk
microbiology source of error
contamination
area of the clear zone may be irregular
microbiology safety precautions
wash hands/sterilise
wear safety goggles with disinfectant
photosynthesis aim
investigate the effect of light intensity on the rate of photosynthesis
photosynthesis method
place 20 algal balls and same amount of indicator solution and replace screw top with correct distance
check the colour of the indicator against the colour chart to measure start pH
leave for hour
record final pH and distance
repeat for more distances
photosynthesis method results
light intensity decreases → rate of photosynthesis decreases → rate of change in pH decreases
photosynthesis controlled variables
temperature
amount of carbon dioxide
volume of indicator solution
photosynthesis hazards
boiling water
respiration practical aim
investigate the rate of respiration in living organisms using a simple respirometer
respiration practical setup
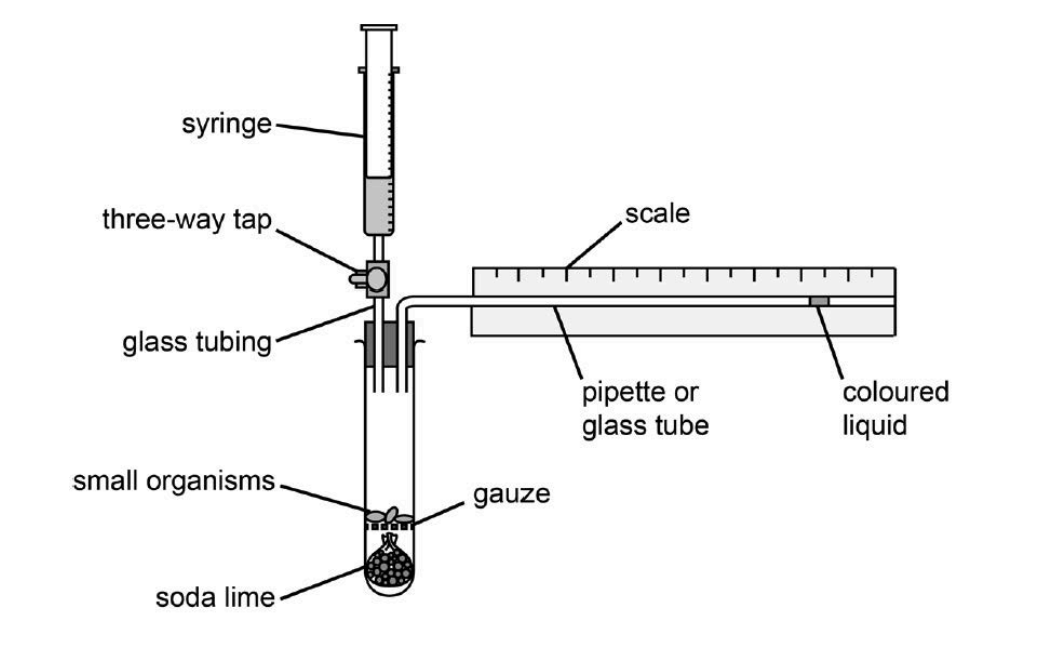
respiration method
set up as in the diagram
weight the organisms and place in boiling tube
open the connection between the syringe and the respirometer
draw the fluid to the end of the scale
leave in a water bath for 5 minutes
mark the start point of the fluid
close the tap to make it airtight and start the timer
record the position of the fluid at one minute intervals for at leave five mins
calculate distance travelled by the fluid per minute
respiration practical controlled variables
mass of soda lime
temperature
time allowed for measuring
respiration practical sources of error
animals may be affected by stress
quadrats aim
estimate the population size of a plant species
transects aim
investigate the effect of variation in a factor on the distribution of plant species
quadrats type of sampling
random sampling
transects type of sampling
continuous sampling
method for quadrats
set up two tape measures on the perimeter of the area
use a random number generator to get two coordinates
place the quadrat at the coordinates
count the number of required plant species
repeat 9 times
population size = total area / area of quadrat x mean number of individuals in a quadrat
method of transects
write down a hypothesis of the effect of a change in an abiotic factor on the distribution of plant species
lay down a tape measure from the base of a tree to an open area
place the quadrat on the 0 end of the tape measure and count the plants
repeat at 5 meter intervals
plot number of plants against the ecological gradient that is observed on the transect line
field investigations controlled variables
size of quadrats
number of repetitions
coordinate system at each site
field investigations sources of error
certain species may be too small to see