Micro + Mesoporous Solids
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
What are porous materials?
materials with a network of atoms containing voids or pores that can selectively trap, adsorb, or react with guest molecules
useful since their pores provide high SA + selectivity for adsorption, storage + reactions
used in both industrial + domestic applications:
gas separation
energy storage
ion exchange
heterogeneous catalysis
green chemistry
detergents
What’s a porous solid?
A solid that contains pores (cavities, channels, or interstices) that are deeper than they are wide.
What’s the difference between microporous, mesoporous + macroporous materials?
microporous: materials with pore diameters less than 2 nm
mesoporous: nanoporous materials with pore diameters between 2-50 nm
macroporous: materials with pore diameters greater than 50 nm
How is porosity (ε) defined + what does it exclude?
ratio of total pore volume (Vp) to the apparent volume (V) of a particle or powder
excludes interparticle voids (spaces between particles)
What is a zeolite?
family of hydrous aluminum silicate minerals (or their synthetic equivalents) that contain cations (Na⁺, K⁺, Ca²⁺, Sr²⁺, Ba²⁺) + are used as molecular filters and ion-exchange agents
pores 4-10 Å + cavities 10-13 Å
structure: crystalline (periodic + regular)
What is a Secondary Building Unit (SBU)?
repeating structural motif formed from linked SiO₄ tetrahedra (ex. 6-membered ring made of 6 SiO₄ units)
topology determines arrangement of pores + channels, influencing molecular transport + adsorption
pore structure can be tailored by altering the SBUs
larger SBUs create larger pores
What happens when Al substitutes for Si in the structure?
creates an aluminosilicate with a net (-) charge that can attract cations or metal dopants
Sn, Hf or Ti added as dopants for catalysis
What happens when Al substitutes for Si in a silica framework?
It introduces a –1 charge that must be balanced by a counterion cation (ex. Na⁺, K⁺, Ca²⁺, Mg²⁺).
How does low Al + high Al concentration affect the structure?
low: minimal effect on the structure + maintains silica-like stability
high: structural distortion increases + more cations are needed to balance charge
How do cation size and charge influence zeolite properties?
They affect pore size, stability, and adsorption capacity — larger cations can block pores.
What is the lowest possible Si/Al ratio?
Si/Al = 1 (equal amounts), but typically Si > Al for stability.
What is the significance of Si/Al > 10?
Minimal structural effect + reduced Al–O–Al linkages due to Al avoidance.
What does the Al avoidance rule mean?
Aluminum atoms prefer not to bond directly to each other (no Al–O–Al linkages).
How does low silica (Si/Al ~1-3) affect properties (ex. Zeolite A & X)?
has lots of Al, so the framework carries many (-) charges → needs many cations to balance
high ion exchange capacity: can easily swap cations like Na⁺, K⁺, Ca²⁺, etc.
very hydrophilic
high acidity due to many Al sites (each Al site acts as a weak acid)
structurally weaker: Al–O bonds are less stable + can be attacked by water or acids
poor thermal stability
How does moderate silica (Si/Al 3-5) affect properties (ex. Modenite & Zeo Y)?
fewer Al sites → less charge → fewer cations needed
improved thermal stability: more Si–O–Si bonds make the structure stronger
lower acidity due to fewer Al sites
more balanced performance: stable + moderately acidic, good for catalysis
How does high silica (Si/Al ~10-100) affect properties (ex. USY, ZSM-5)?
very little Al → almost neutral framework (few negative charges)
hydrophobic
very thermally stable: strong Si–O–Si bonds resist heat
no ion-exchange capacity: very few cations present
low acidity: few active sites left for catalysis.
good for hydrocarbon adsorption + catalytic cracking (ex. in fuels)
How does pure silica (Si/Al = ∞) affect properties (ex. silicalite)
no Al
completely neutral + hydrophobic
extremely thermally + chemically stable
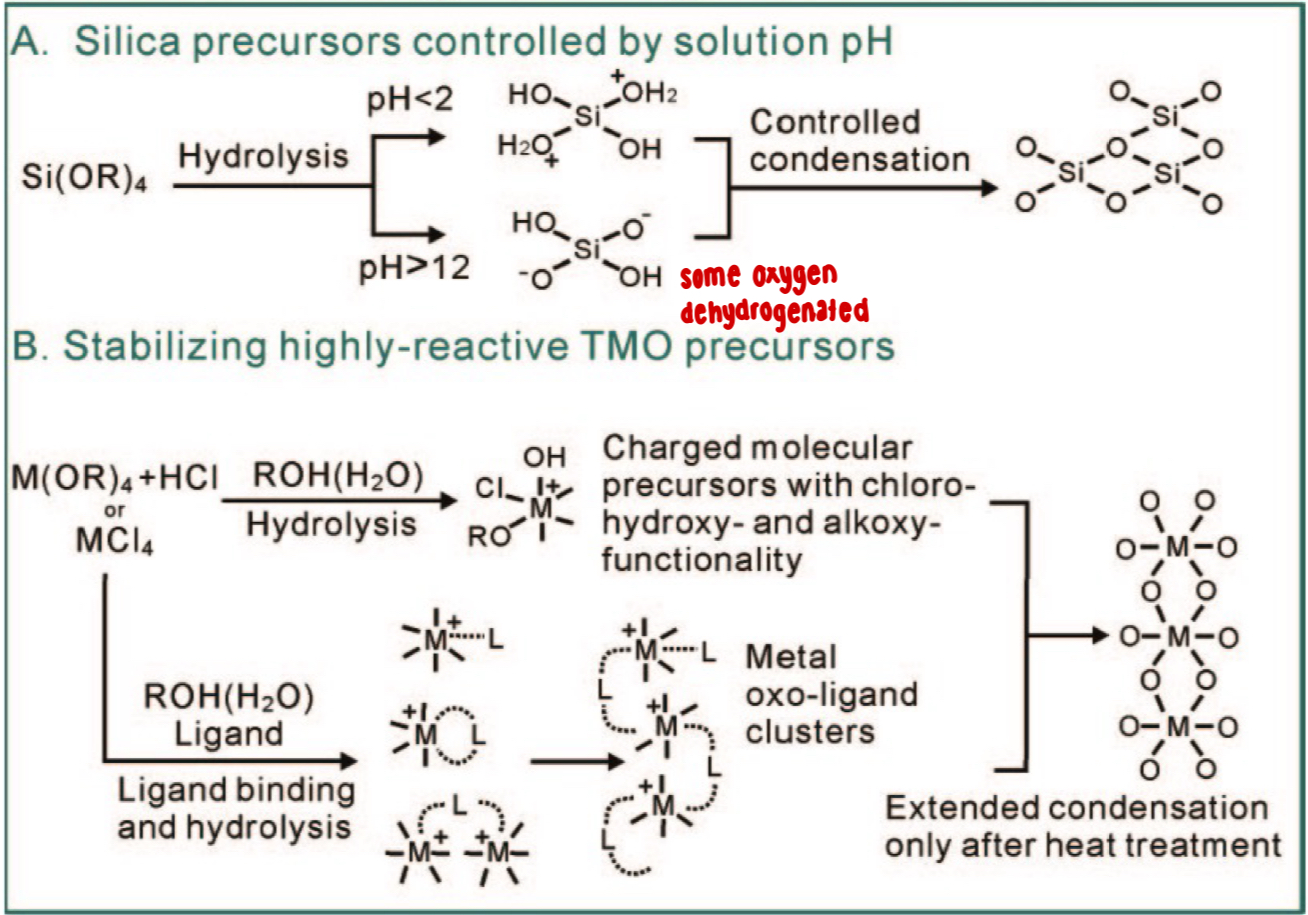
Can you explain how stable charged inorganic precursors are formed during the sol-gel process?
formed through hydrolysis + condensation reactions
silica precursors (Si(OR)₄):
under acidic conditions (pH < 2): hydrolysis forms (+) charged silanol species
under basic conditions (pH > 12): hydrolysis forms (-) charged silanol species
these intermediates undergo condensation reactions (–OH + –OH → –O– + H₂O), gradually forming Si–O–Si linkages
some O atoms are dehydrogenated ➡ growing silica network
transition metal oxide (TMO) precursors (M(OR)₄ or MCl₄):
hydrolysis with HCl or ROH(H₂O) forms charged molecular precursors containing chloro-, hydroxy- + alkoxy-functionalities
ligands (alcohols, water) coordinate with the metal, stabilizing reactive centers + preventing premature condensation
controlled ligand binding + hydrolysis form small metal oxo-ligand clusters (M–O–M)
extended condensation occurs only after heat treatment, removing ligands + producing a continuous metal–oxygen–metal (M–O–M) solid network
In the sol-gel process, what is the role of the surfactant/amphiphile in the 2nd step: molecular interactions?
interacts with metal ion precursors, helping organize the ions + solvent to guide structured network or pore formation in the material
important to choose correct surfactant since it ensures effective interaction with the metal precursor, which controls the nanostructure + porosity of the final material
What characterizes the hydrophobic + hydrophilic part of a surfactant in the sol-gel process?
hydrophobic: hydrocarbon tail or a carbon-rich polymer block
it avoids water + prefers non-polar environments
hydrophilic: charged head group or an oxygen-rich polymer block
interacts with water + polar species, including metal ions
What is the main goal of the mesostructure synthesis in the sol-gel process?
To create conditions under which the surfactant can self-assemble, guiding the formation of an ordered inorganic mesoporous framework.
What are the stages of the 3rd step: mesostructure synthesis in the sol-gel process?
molecular interactions
co-assembly of surfactant + metal species
partially condensed mesostructured hybrid
inorganic network starts forming but is not fully rigid; the surfactant template is still present, maintaining the structure
inorganic mesoporous framework
surfactant is removed
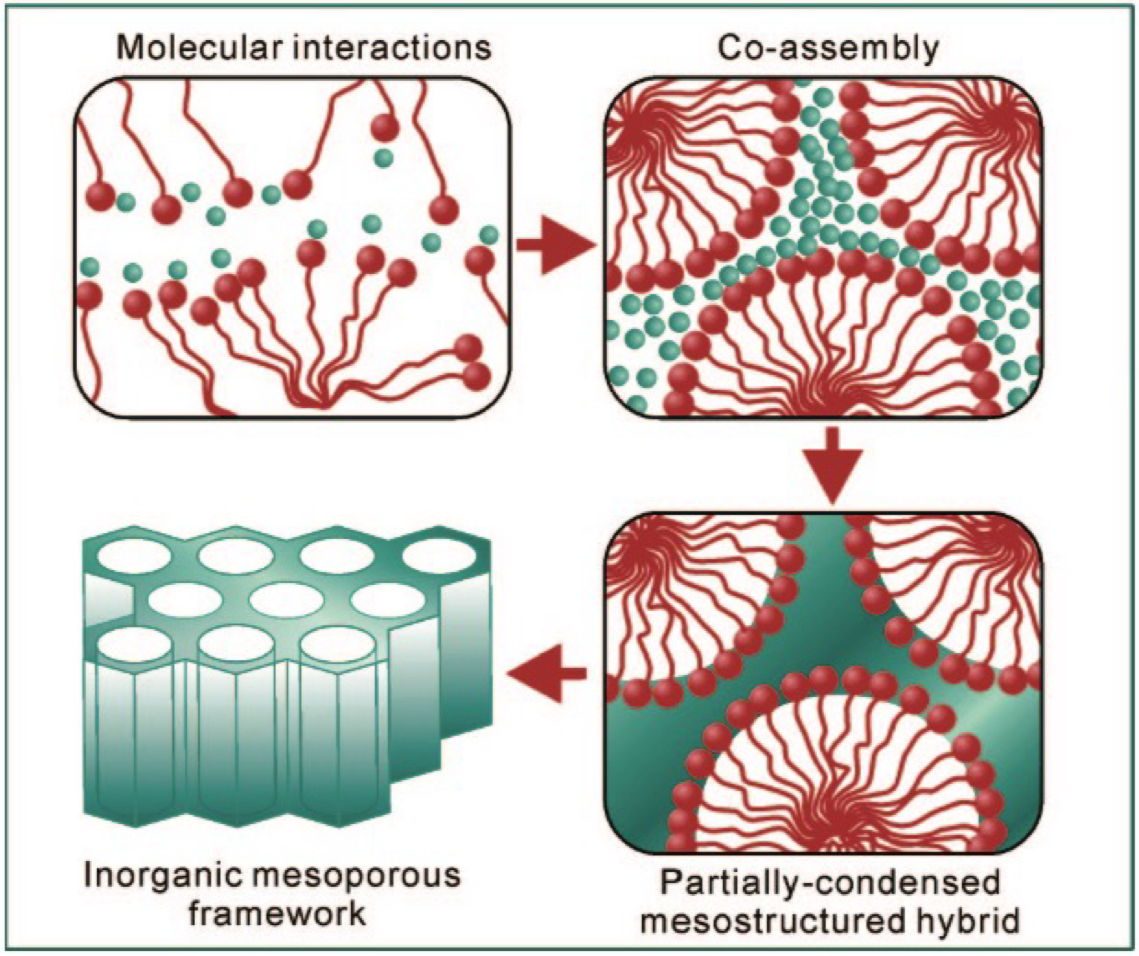
Why is controlling the self-assembly conditions important in the sol-gel process?
Because the pore size, shape + order of the final mesoporous material depend on how the surfactant + metal precursors co-assemble.
What types of non-covalent interactions drive self-assembly?
H-bonding, van der Waals forces + electrostatic interactions.
What happens during the “bake” step of the sol-gel process?
heating removes the organic surfactants (burns them to CO₂) + dehydrates the metal sol-gel, forming a hard, rigid inorganic oxide
sol-gel material is “hard as a rock” since metal oxide network condenses + loses water
What are inorganic-organic (I/O) hybrid materials?
materials that combine inorganic + organic components at the molecular level, often prepared via the sol-gel process
steps:
formation of sol
inorganic + organic precursors are dissolved or dispersed to form a stable colloidal solution
crosslinking of the sol
precursor molecules are chemically bonded to form gel
aging
gel network strengthens + evolves as condensation reactions continue
drying
solvent + byproducts are removed, yielding the final solid hybrid material
What factors influence the properties of I/O hybrids?
chemical composition of precursors
ratio of inorganic to organic components
structure of building blocks
distribution of building blocks
How are organic molecules incorporated into sol-gel materials?
they’re dissolved in the sol-gel precursor solution + the gel forms around them
physically trapped but not covalently bonded ➡ retains their functionality
Why is sol-gel processing suitable for embedding biomolecules?
mild, allowing sensitive biomolecules to be incorporated without denaturation
stabilizes the molecules, preserving their function for applications
What does covalent modification in sol-gel hybrids involve?
attaching organic R groups to the inorganic sol-gel network, typically via organosilane precursors
modifies networks polarity + chemical environment, influencing hydrophobicity + flexibility
reduces crosslinking because the R groups occupy sites that would otherwise form Si–O–Si bonds
Why use covalent modification in sol-gel materials?
To tailor the physical + chemical properties of the hybrid material by choosing specific organic groups.
How do R substituents affect connectivity in sol-gel networks?
They reduce the number of reactive sites per silicon, changing connectivity + often preventing fully cross-linked 3D networks
What type of structure is formed using di-substituted or mono-substituted precursors?
di-substituted: linear systems
mono-substituted: cage systems (POSS)
Why is it difficult to prepare 3D gel networks from organically substituted precursors?
because the organic R groups reduce the number of bonding sites
fully crosslinked networks are more easily formed with tetraalkoxysilanes (Si(OR)₄)
How do bulkier R groups influence the reaction?
They can sterically slow the reaction, but electronic effects are usually more significant in controlling hydrolysis.
What is a major challenge when using mixtures of organically modified sol-gel precursors?
phase segregation: different precursors separate instead of forming a homogeneous gel
occurs since TS of hydrolysis + condensation reactions differ between precursors ➡ uneven reaction rates
Why is pH important in sol-gel precursor mixtures?
It affects hydrolysis + condensation rates, which influence the structure, uniformity + potential phase segregation of the gel.
What is Rw in sol-gel chemistry?
The alkoxy-to-water ratio: moles of water per mole of alkoxy groups in the precursor.
How does Rw ≤ 2 affect the sol-gel reaction VS Rw >> 2 VS Rw = 1?
Rw ≤ 2: favours hydrolysis, forming Si–OH groups over Si–O–Si condensation
Rw >> 2: favours condensation, forming Si–O–Si linkages + promoting network formation
Rw = 1: full hydrolysis with minimal condensation
Why does excess water make condensation less likely?
Because more water molecules reduce the likelihood that Si–OR groups encounter each other, making reaction with water more probable.
What happens at the gel point + are chemical reactions stopped?
viscosity increases, freezing the network (glass-forming process)
hydrolysis + condensation continue, allowing further chemical evolution of the gel
What is an alcogel + how does it affect aging?
alcogel: gel with a continuous liquid phase containing solvent, particles + unreacted monomers
allows ongoing condensation of M–OH + M–OR groups, leading to network densification, shrinkage (syneresis) + structural rearrangements
What processes occur during ripening/coarsening in aging + what are their effects?
material redistributes from thermodynamically unfavourable regions via reversible hydrolysis/condensation
effects:
reduces net curvature
removes small particles
fills pores (analogous to sintering)
optimizes porosity, network strength + mechanical properties
What are the 3 main stages of drying a sol-gel?
shrinkage stage:
gel volume decreases as liquid flows from core to surface
flexible networks deform + OH groups can react
pore size shrinkage increases surface tension
rigid network stage:
surface tension can no longer deform the network
shrinkage stops + cracking may occur
liquid/gas interface retreats, leaving continuous liquid film on pore walls
diffusion stage:
liquid film ruptures
remaining liquid exists in isolated regions + leaves the network via gas-phase diffusion
What is Ostwald ripening + what factors influence particle growth in sol-gel systems?
process where small particles dissolve + redeposit onto larger ones, favouring the formation of larger particles over time
growth stops when solubility differences between small + large particles are minimal
factors:
pH: silica grows to 5–10 nm when pH > 7; 2–4 nm at lower pH
T: higher T → larger particles + system dynamics (ex. ouzo effect)
How does the Stöber method control silica particle size and morphology?
TEOS reacts in a basic solution (ammonia catalyst) with excess water
particle size is mainly controlled by T + reactant concentrations
silica spheres form through nucleation + growth, followed by cycles of dissolution + re-precipitation
this rate-limited monomer–cluster growth keeps monomer levels steady, producing uniform + well-defined particles
What is Rate-Limited Monomer Cluster Growth in Stöber silica?
growth of silica spheres is controlled by how quickly monomeric TEOS-derived species attach to existing clusters
this slow monomer addition allows repeated nucleation, dissolution + re-precipitation
produces uniform, monodisperse silica particles
What causes network collapse + cracking in sol-gel materials?
slower shrinkage of the interior results in pressure gradient that causes cracking
larger pores will empty faster than small pores, causing uneven stress on walls + cracking
Whats the difference between xerogel powder + aerogels?
xerogel powder:
formed when gels are conventionally dried
cracking can be minimized but shrinkage is inevitable
aerogels:
formed by replacing solvent with a gas
causes little or no change to network structure or volume
How do square planar copper nodes + tritopic linkers determine MOF structures, and what role does the node-to-linker ratio play?
square planar copper nodes act as corners
tritopic linkers connect 3 nodes
controlling the node-to-linker ratio tunes connectivity + mesoporous crystal topology
resulting MOFs have tailored pore sizes, shapes + functionalities for catalysis, gas storage + separations