Chemistry OCR - Module 4: Section 1 Basic Concepts and Hydrocarbons
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
70 Terms
What are the different types of formulae and what do they show? (6)
General Formula - An algebraic formula that can describe any member of a family of compounds.
Empirical Formula - The simplest whole number ratio of atoms of each element in a compound.
Molecular Formula - The actual number of atoms of each element in a molecule.
Structural Formula - Shows the arrangement of atoms carbon by carbon.
Skeletal Formula - Shows the bonds of the carbon skeleton only, with any functional groups.
Displayed Formula - Shows how all the atoms are arranged.
What are a homologous series?
Is a bunch of organic compounds that have the same functional group and general formula. Differs by CH2
What is a functional group?
A group of atoms in a molecule responsible for the characteristic reactions of that compound.
What are the different types of homologous series? (10)
Alkanes
Branched alkanes
Alkenes
Haloalkanes
Alcohols
Aldehydes
Ketones
Cycloalkanes
Carboxylic Acids
Esters
What is the Alkane homolgous series?
Prefix or suffix -ane
e.g. Propane
What is the branched alkanes homologous series?
Prefix or suffix - alkyle-(-yl)
e.g. methylepropane
What is the alkene homologous series?
Prefix or suffix - ene
e.g. propene
What is the haloalkanes homologous series?
Prefix or suffix - chloro-/bromo-/iodo-
Chloroethane
What is the alcohols homologous series?
Prefix or suffix - ol
e.g. ethanol
What is the aldehydes homologous series?
Prefix or suffix - al
e.g. ethanal
What is the ketone homologous series?
Prefix or suffix - one
e.g. Propanone
What is the cycloalkane homologous series?
Prefix or suffix - cyclo-…-ane
Cyclohexane
what is the carboxylic acid homologous series?
Prefix or suffix - oic acid
e.g. ethanoic acid
What is the esters homologous series?
Prefix or suffix - alkyl-…-anoate
e.g. methyl propanoate
What is an aromatic carbon skeleton?
Aromatic compounds contain a benzene ring.
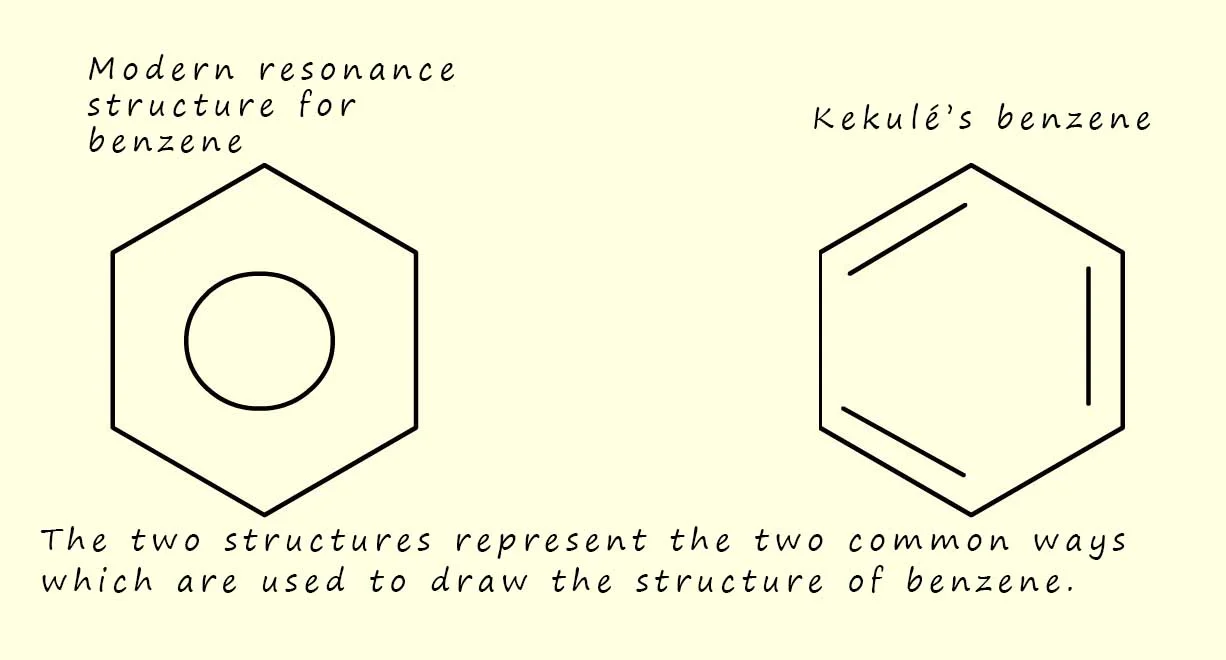
What is an aliphatic carbon skeleton?
Aliphatic compounds contain carbon and hydrogen joined together in straight chains, branched chains or non-aromatic rings.
What is an alicyclic carbon skeleton?
If an aliphatic compound contains a (non-aromatic) ring, then it can be called alicyclic.
Or without side chains
What is a saturated and unsaturated compound?
Saturated compounds only contain carbon-carbon single bonds, like alkanes.
Unsaturated compounds can have carbon-carbon double bonds, triple bonds or aromatic groups.
What is an alkyl group?
An alkyl group is a fragment of a molecule with general formula CnH2n+1
How do you name organic compounds using IUPAC Nomenclature?
Count the carbon atoms in the longest continuous chain.
The main functional group of the molecule usually tells you what homologous series the molecule is in, and so gives you the prefix or suffix.
Number the longest carbon chain so that the main functional group has the lowest possible number. If there is more than one longest chain, pick the one with the most side-chains.
Any side-chains or less important functional groups are added as prefixes at the start of the name. Put them in alphabetical order, after the number of the carbon atom each is attached to.
If there is more than one identical side-chain or functional group, use di-, tri-, or tetra- before that part of the name, but ignore this when working out the alphabetical order.
What are the first 10 alkanes?
Methane
Ethane
Propane
Butane
Pentane
Hexane
Heptane
Octane
Nonane
Decane
What are isomers and what are the two branches of isomers?
Two molecules are isomers of one another if they have the same molecular formula but the atoms are arranged differently.
Structural isomers and Stereoisomers
What are the different Structural isomers?
Chain isomers
Position isomers
Functional group isomers
What is a structural isomer?
In structural isomers, the atoms are connected in different ways. So although the molecular formula is the same, the structural formula is different.
What is a chain isomer?
The carbon skeleton can be arranged differently.
These isomers have similar chemical properties, but their physical properties, like boiling point, will be different because of the change in shape of the molecule.
What are positional isomers?
The skeleton and the functional group could be the same, only with the functional group attached to a different carbon atom.
These also have different physical properties, and the chemical properties might be different too.
What are functional group isomers?
The same atoms can be arranged into different functional groups.
These have very different physical and chemical properties.
What are alkanes and what is their general formula?
Alkanes are hydrocarbons.
Alkanes are saturated hydrocarbons containing single C-C and C-H bonds as σ-bonds and have free rotation around the σ-bonds.
CnH2n+2
What is the shape of alkane molecules around each carbon atom?
Tetrahedral, 109.5 degrees
This is because each carbon atom has four pairs of bonding electrons around it. They all repel each other equally.
Why do alkanes have different boiling points depending on their size and shape?
Alkanes have covalent bonds inside the molecules. Between the molecules, there are induced dipole-dipole interactions which hold them all together.
The longer the carbon chain, the stronger the induced dipole-dipole interactions. This is because there is more surface contact and more electrons to interact.
As the molecules get longer, it takes more energy to overcome the induced diple-dipole interaction, and the boiling point rises.
A branched-chain alkane has a lower boiling point than its straight-chain isomer. Branched-chain alkanes cannot pack closely together and they have smaller molecular surface areas, so the induced dipole-dipole interactions are reduced.
What is the combustions of alkanes like?
Combustion reactions happen between gases, so liquid alkanes have to be vaporised first. Smaller alkanes turn into gases more easily, they are more volatile, so they will burn more easily too.
Larger alkanes release heaps more energy per mole because they have more bonds to react.
Because they release so much energy when they burn, they are great fuels.
What happens if alkanes are burned with not enough oxygen?
Carbon monoxide will be produced with is poisonous as it binds to you haemoglobin better than oxygen causing oxygen deprivation in your body.
What is bond fission?
Breaking a covalent bond is called bond fission.
What are the two types of bond fission?
Heterolytic fission
Homolytic fission
What is Heterolytic Fission?
In heterolytic fission the bond breaks unevenly with one of the bonded atoms receiving both electrons from the bonded pair. Two different substances are formed, a positively charged cation, and a negatively charged anion
What does a curly arrow show?
The movement of an electron pair
What is Homolytic Fission?
In homolytic fission, the bond breaks evenly and each bonding atom receives one electron from the bonded pair. Two electrically uncharged ‘radicals’ are formed. Because of the unpaired electron, radicals are very reactive.
What is a radical?
Radicals are particles that have an unpaired electron. They are shown in mechanisms by a big dot next to the molecular formula.
Where do halogens react with alkanes?
Halogens react with alkanes in photochemical reactions. Photochemical reactions are started by light, this reaction requires ultraviolet light to get going.
What type of reaction is a reaction between alkanes and halogens?
Free-radical substitution reaction
What are the three stages of the reaction mechanisms for the reaction between chlorine and methane?
Initiation reactions
Propagation reactions
Termination reactions
What is the initiation reaction for the reaction between chlorine and methane?
Sunlight provides enough energy to break the Cl-Cl bond, this is photodissociation
Cl2→2Cl∙
The bond splits equally and each atom gets to keep one electron - homolytic fission. The atom becomes a highly reactive free radical, Cl∙, because of its unpaired electron.
What are the propagation reactions in the reaction between chlorine and methane?
Cl∙ attacks a methane molecule: Cl∙ + CH4 → ∙CH3 + HCl
The new methyl free radical, ∙CH3, can attack another Cl2, molecule: ∙CH3 + Cl2 → CH3Cl + Cl∙
The new Cl∙ can attack another CH4 molecule, and so on, until all the Cl2 or CH4 molecules are wiped out.
What are the termination reactions in the reaction between chlorine an methane?
If two free radicals join together, they make a stable molecule.
There are heaps of possible termination reactions.
Cl∙ + ∙CH3 → CH3Cl
∙CH3 + ∙CH3 → C2H6
What are the limitations of free-radical substitution?
You get a mixture of products.
e.g. If you are trying to make chloromethane, and there is too much chlorine in the reaction mixture, some of the remaining hydrogen atoms on the chloromethane molecule will be swapped for chlorine atoms. The propagation reactions happen again this time to make dichloromethane.
Cl∙ + CH3Cl → ∙CH2Cl + HCl
∙CH2Cl + Cl2 → CH2Cl2 + Cl∙
Another substitution reaction can take place to form trichloromethane
Cl∙ + CH2Cl2 → ∙CHCl2 + HCl
∙CHCl2 + Cl2 → CHCl3 + Cl∙
Tetrachloromethane is formed in the last possible substitution.
This is a nuisance because you have to separate the chloromethane from the other three unwanted by-products.
The best way of reducing the chance of these by-products forming is to have excess of methane. This means there is a greater chance of a chlorine radical colliding only with a methane molecule and not a chloromethane molecule.
Another problem with free radical substitution is that it can take place at any point along the carbon chain. So a mixture of isomers can be formed.
What are alkenes and what is their general formula?
CnH2n
They are made of carbon and hydrogen atoms, so they are hydrocarbons.
Alkene molecules all have at least one C=C double covalent bond. Molecules with C=C double bonds are unsaturated because they can make more bonds with extra atoms in addition reactions.
What is a double bond made up of?
A σ sigma bond and a π pi bond
What is a σ bond?
Formed when two s orbitals overlap.
The two s orbitals overlap in a straight line, this gives the highest possible electron density between the two nuclei. This is a single covalent bond.
The high electron density between the nuclei means there is a strong electrostatic attraction between the nuclei and the shared pair of electrons. This means that σ bonds have a high bond enthalpy. They are the strongest type of covalent bonds.
What is a π bond?
Formed by the sideways overlap of two adjacent p orbitals.
It has got two parts to it, one ‘above’ and one ‘below’ the molecular axis. This is because the p orbitals which overlap are dumb-bell shaped.
π bonds are much weaker than σ bonds because the electron density is spread out above and below the nuclei. This means that the electrostatic attraction between the nuclei and the shared pair of electrons is weaker, so π bonds have relatively low bond enthalpy.
Why are Alkenes more reactive than akanes?
Alkanes only contain C-C and C-H σ bonds, which have a high bond enthalpy and so are difficult to break. The bonds are also non-polar so they do not attract nucleophiles or electrophiles.
Alkenes are more reactive than alkanes because the C=C bond contains both a σ bond and a π bond.
The C=C double bond contains four electrons so it has a high electron density and the π bond also sticks out above and below the rest of the molecule. These two factors mean the π bond is likely to be attacked by electrophiles. The low bond enthalpy of the π bond also contributes to the reactivity of alkenes.
Because the double bond’s so reactive, alkenes are handy starting points for making other organic compounds and for making petrochemicals.
Why do double bonds not rotate?
C=C bonds are said to be trigonal planar, so the C=C and all other atoms lie on the same plane.
The atoms cannot rotate around them like they can around single bonds because of the way the p orbitals overlap to form a π bond. The do not bend much either.
Even though atoms cannot rotate, about the double bond, things can still rotate about any single bonds in the molecule.
The restricted rotation around C=C double bond is what causes alkenes to form stereoisomers.
What are stereoisomers?
Same structural formula but a different arrangement in space. Due to the lack of rotation around the double bond, some alkenes can have some stereoisomers.
When do Stereoisomers occur?
When two double-bonded carbon atoms each have two different atoms or groups attached to them.
What are the different types of stereoisomers?
E-isomer - has the same groups positioned across the double bond.
Z-isomer - has the same groups either both above or both below the double bond.
How does the E/Z system in stereoisomers work even when all the groups are different?
Using Cahn-Ingold-Prelog rules.
Atoms with a larger atom number are given higher priority:
Look at the atoms directly bonded to each of the C=C atoms, the atom with the higher atomic number on each carbon is given the higher priority.
Be careful if you are doing this for an alkene with only 3 different groups. The E/Z system gives the positions of the highest priority group on each carbon, which are not always the matching groups.
You may have to look further along the chain:
If the atoms directly bonded to the carbon are the same then you have to look at the next atom in the groups to work out which has the higher priority.
What are Cis-Trans Isomers?
If the carbon atoms have at least one group in common, then you can call the isomers, ‘cis’ or ‘trans’ where
‘cis’ means the same groups are on the same side of the double bond
‘trans’ means the same groups are on opposite sides of the double bond
If the carbon atoms both have totally different groups attached to themm the cis-trans naming system cannot cope.
What is electrophilic addition?
The alkene double bond opens up and atoms are added to the carbon atoms.
Electrophilic addition reactions happen because the double bond has got plenty of electrons and is easily attacked by electrophiles.
What are electrophiles?
Electrophiles are electron-pair acceptors, they are usually a bit short of electrons, so they are attracted to areas where there are lots of them about.
Electrophiles include positively charged ions, like H+ and NO2+, and polar molecules.
What is formed when hydrogen reacts with C=C bonds and what is required for this to happen?
It will react with hydrogen gas to produce alkanes.
It needs a nickel catalyst and a temperature of 150°C
How will Halogens react with alkenes to form dihaloalkanes?
Halogens will react with alkenes to form dihaloalkanes, the halogens add across the double bond, and each of the carbon atoms ends up bonded to one halogen atom. It is an electrophilic addition reaction.
You can use bromine water to test for carbon double bonds
When you shake an alkene with orange bromine water, the solution quickly decolourises.
This is because bromine is added across the double bond to form a colourless dibromoalkane.
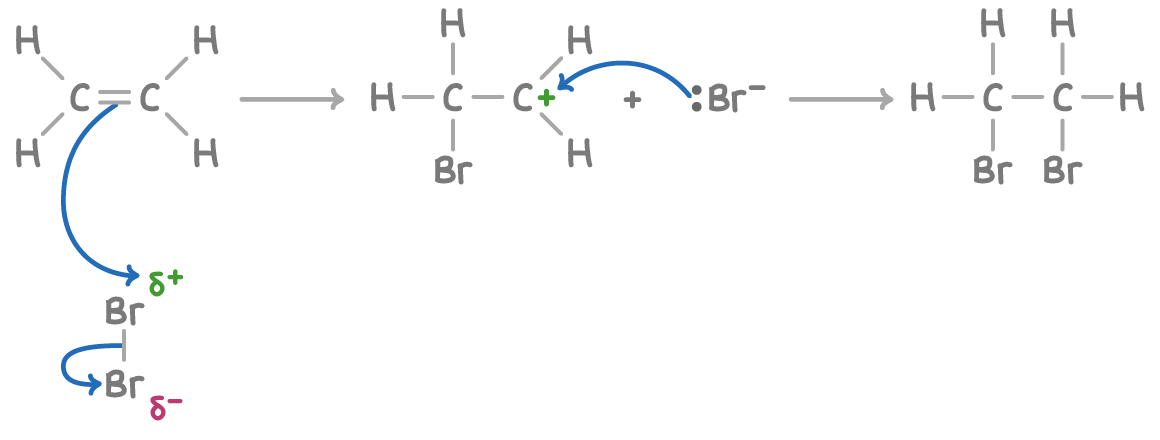
(DESCRIPTION OF REACTION MECHANISM)
The double bond repels the electrons in Br2, polarising the Br-Br.
Heterolytic fission of Br2. The closer Br gives up the bonding electrons to the other Br and bonds to the C atom.
You get a positively charged carbocation intermediate. The Br- now moves…
And bonds to the other Carbon, forming 1,2-dibromoethane.
How can alcohols be make by steam hydration?
Alkenes can be hydrated by steam at 300°C and a pressure of 60-70atm. The reaction needs a solid phosphoric(V) acid catalyst (H3PO4)
The reaction is used to manufacture ethanol from ethene.
The reaction is reversible and the reaction yield is low, with ethene it is only about 5%. You can recycle the unreacted alkene gas, making the overall yield much better, you can get a yield of 95% with ethene.
What is a carbocation?
A carbocation is an organic ion containing a positively charged carbon atom.
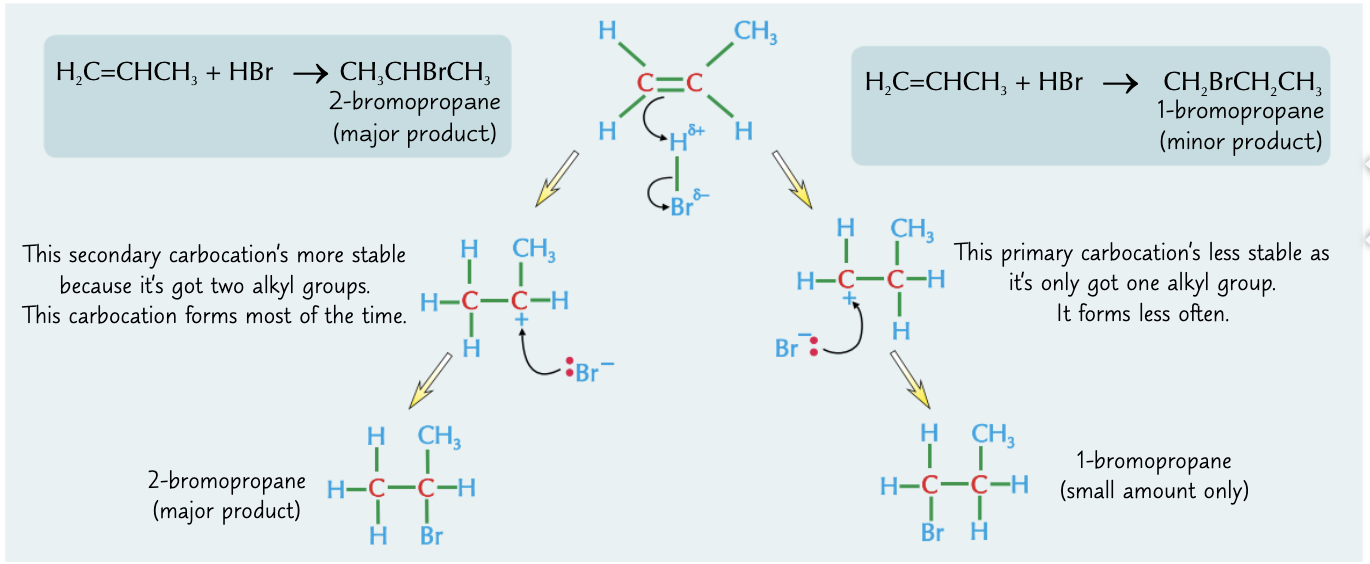
How can you add hydrogen halides to unsymmetrical alkenes to form two products?
If the hydrogen halide adds to an unsymmetrical alkene, there are two possible products.
The amount of each product depends on how stable the carbocation formed in the middle of the reaction is.
Carbocations with more alkyl groups are more stable because the alkyl groups feed electrons towards the positive charge. The more stable carbocation is much more likely to form.
The least stable to most stable is as follows, primary carbocation < secondary carbocation < tertiary carbocation
What is Markownikoff’s rule?
The major product from addition of a hydrogen halide (HX) to an unsymmetrical alkene is the one where hydrogen adds to the carbon with the most hydrogens already attached.
Describe the reaction and reaction mechanism of how hydrogen bromide reacts with propene.
How can Alkenes join up to form addition polymers?
The double bonds in alkenes can open up and join together to make long chains called polymers.
This is called addition polymerisation
To find the monomer used to form an addition polymer, take the repeat unit and add a double bond.
Why are polymers useful?
They are very unreactive. So food does not react with the PTFE coating on pans, plastic windows do not rot etc.
Why are polymers a problem.
The lack of reactivity also leads to a problem. Most polymers are not biodegradable, and so they are really difficult to dispose of.
In the UK over 2 million tonnes of plastic waste are produced each year. It is important to find ways to get rid of this waste while minimising environmental damage.
How is plastic waste being reduced?
Waste plastic can be buried.
Landfill is one option for dealing with waste plastics. It is generally used when plastic is:
difficult to separate from other waste
not in sufficient quantities to make separation financially worthwhile
too difficult technically to recycle.
But because the amount of waste we generate is becoming more and more of a problem, there is a need to reduce landfill as much as possible.
Waste plastics can be reused.
Many plastics are made from non-renewable oil-fractions, so it makes sense to reuse plastics as much as possible.
After sorting into different types:
some plastics can be recycled by melting and remoulding them
some plastics as an organic feedstock to make more plastics or other chemicals.
Waste plastics can be burned:
If recycling is not possible, waste plastics can be burned, and the heat can be used to generate electricity.
This process needs to be carefully controlled to reduce toxic gases. for example, polymers that contain chlorine (such as PVC) produces HCl when they are burned - this has to be removed.
Waste gases from the combustion are passed through scrubbers which can neutralise gases such as HCl by allowing them to react with a base.
What are the benefits of the development of biodegradable and photodegradable polymers?
Biodegradable polymers decompose pretty quickly in certain conditions because organisms can digest them.
Biodegradable polymers can be made from renewable raw materials such as starch or oil fractions, such as from the hydrocarbon isoprene. But at the moment they are more expensive than non-biodegradable equivalents.
Even though they are biodegradable, these polymers still need the right conditions before they will decompose. You could not necessarily just put them in a landfill and expect them to perish away because there is a lack of moisture and oxygen under all that compressed soil. You need to chuck them on a big compost heap.
This means that you need to collect and separate the biodegradable polymers from non-biodegradable plastics.
There are various potential uses, e.g. plastic sheeting used to protect plants from the frost can be made from poly(ethene) with starch grains embedded in it. In time the starch is broken down by microorganisms and the remaining poly(ethene) crumbles into dust. There is no need to collect and dispose of the old sheeting.
Scientists have also started developing photodegradable polymers. These are polymers that decompose when exposed to sunlight.