Cell-Cell communication
1/85
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
86 Terms
Why cell-cell signaling important?
Cell need to be able to interact with their environment another cells around them to function properly
Communication via cells is mainly carried out via extracellular/intracellular signal molecules
extracellular
What are the steps of cell signaling?
1. Reception
2. Transduction
3. Response
T/F Cell signals only occur when cells are in close proximity
False. They can signal over short or long ranges
T/F The same signal can have different response on different cells
True
What are the types of chemical signaling between cells?
- cytoplasmic connections
- free diffusion
- cell-to-cell contact mediated (via ligands)
Transmission of signals through gap junctions is what kind of signaling?
signaling via cytoplasmic connections (can readily move between cell junctions)
Free diffusion is the best method of transportation for what type of cell signaling?
- hormones
- adjacent cells (via interstitial space)
What are the types of ligand intercellular signal?
- contact dependent
- paracrine
- synaptic
- endocrine
How does contact dependent signaling take place?
- the signaling cell has a membrane bound protein and the target cell has a a membrane bound receptor.
- when the two cells are within close proximity to each other, the protein on the signaling cell binds to the receptor on the large cell and a signal is transduced
How does paracrine signaling work?
- via local diffusion
- when the signaling cell is within close proximity with the receptor cells, the signaling protein is released
- the proteins then bind to the receptors and a signal is transduced
Autocrine vs Paracrine
- autocrine signals come from within the cell and bind to receptors on the outside of the SAME cell to transduce a signal
- paracrine signals come from nearby cells and bind to the receptors of the target cells
How does synaptic signaling work?
- via local diffusion
- neurotransmitters are release from the pre-synaptic neuron into the synaptic cleft to bind with the target post-synaptic neuron
How is neuronal signaling similar/different from endocrine signaling?
similar: it uses paracrine signaling AND one cell can affect multiple target cels
difference: neuronal signaling only occur in neurons, which form synaptic clefts between signaling and target cells
How does endocrine signaling work?
- via long distance diffusion
- hormones are released from the endocrine neurons (which are typically not near the cells that are their targets)
- they are carried through the blood where they bind with the receptors of the target cells
T/F Signal protein are very specific
True
Why is it important that endocrine signal proteins are specific?
endocrine signals typically have to travel through the blood to reach there target cells. because they will encounter numerous amount of cells, it is important that they only bind to a specific cell so that the correct signal can be sent
What are the types of molecules that can be ligands?
- proteins
- small peptide
- amino acids
- steroids
- fatty acid derivatives
- dissolved gases
- etc
Receptor cells on target proteins are what kind of membrane proteins?
integral
Ligands that bind to cell surface receptors are hydrophobic/hydrophilic
hydrophilic (hydrophobic molecules diffuse directly into the cell)
Cells respond to multiple/one cellular signal(s).
- multiple
- cells respond to specific combinations of signals in order to cary out specific actions (ex. survival require a specific combination of signals, whereas growing/dividing and differentiation both require a different set of signals)
What occurs if a cell cannot receive and transmit signals properly?
cell death (apoptosis)
How do ligand-binding receptors work?
- a ligand binds to the surface protein
- the proteins converts the extracellular signal into an intracellular signal
- the signal then is carried into the inside of the target cell to alter the behavior
T/F Cell-surface receptors participate in both extracellular AND intracellular signaling
True.
- ligands bind to the extracellular surface of the protein and transduce that signal into one that can be sent intracellularly
- the cytoplasmic side of the receptor protein then receives the signal, and send it to the appropriate location for the signal to be received
How does the intracellular signal transmit the extracellular signal?
once the signal is received on the cytoplasmic side of the receptor protein, it either:
- recruits secondary messengers (like cAMP or Ca2+) OR
- it activates specific effector proteins
What are secondary messengers?
they are generally specific proteins (since there is a lot of molecules to bind to in the cytoplasm) that behave like molecular switches
Why are second messengers important?
they are typically generated in large amounts which makes the spreading of signals faster and more efficient
What are the types of molecular switches?
- signaling via phosphorylation
- signaling via GTP binding
What are the steps of a molecular switch signaling via phosphorylation?
- the molecular switch is off
- a signal is sent into the cell
- a protein kinase converts ATP into ADP, and phosphorylates the molecular switch
- the phosphorylation of the molecular switch turns it on, and the desired product is made
- when the signal stops transmitting, a protein phosphatase removes the phosphate groups from the molecular switch, which turns it off
What are the steps of a molecular switch signaling via GTP-binding?
- a GDP molecule is bound to the molecular switch, which keeps it in an off position
- when a signal is transduced into the cell, Guanine nucleotide exchange factor (GEF) removed GDP from the molecular switch, and replaces it with GTP
- GTP turns the switch on, and the desired product is made
- When the signal is no longer transduced into the cell, GTPase activating protein (GAP) removes a phosphate from the ATP and converts it into GDP.
How are signals transduced inside the cell if second messengers aren't used?
via signaling complexes
How do signaling complexes work?
- once the signal is inside the side, scaffold proteins are recruited
- the scaffold proteins bring together the groups of the interacting proteins so that specific interactions can take place
What are the types of signaling complexes that can be formed?
- pre-formed signaling complex on a scaffold protein
- signaling complex on phosphoinositides
- complex assembly on activated proteins
How do pre-formed complexes transmit signals into the cell?
- the intracellular signaling proteins are already attached to scaffold protein, which is attached to the inactive receptor
- when a ligand bind to the receptor, the signal is transmitted across the cell membrane
- the complex receives the cell, which then activates the protein bound to the scaffold one by one to activate the target protein to make the desired product

How do phosphoinositide docking sites transmit signals?
- the receptor and the phosphoinositides are within close proximity of each other and the signaling proteins are in the cytoplasm
- the receptor starts off as inactive and the phosphoinositides have two phosphate groups attached
- when a ligand binds to the receptor, it causes the phosphorylation of the phosphoinositides
- when the phosphoinositidse are phosphorylated, the intracellular signaling proteins bind
- once the intracellular proteins bind, the form a dimer, and the signal can be transmitted so the desired product can produced
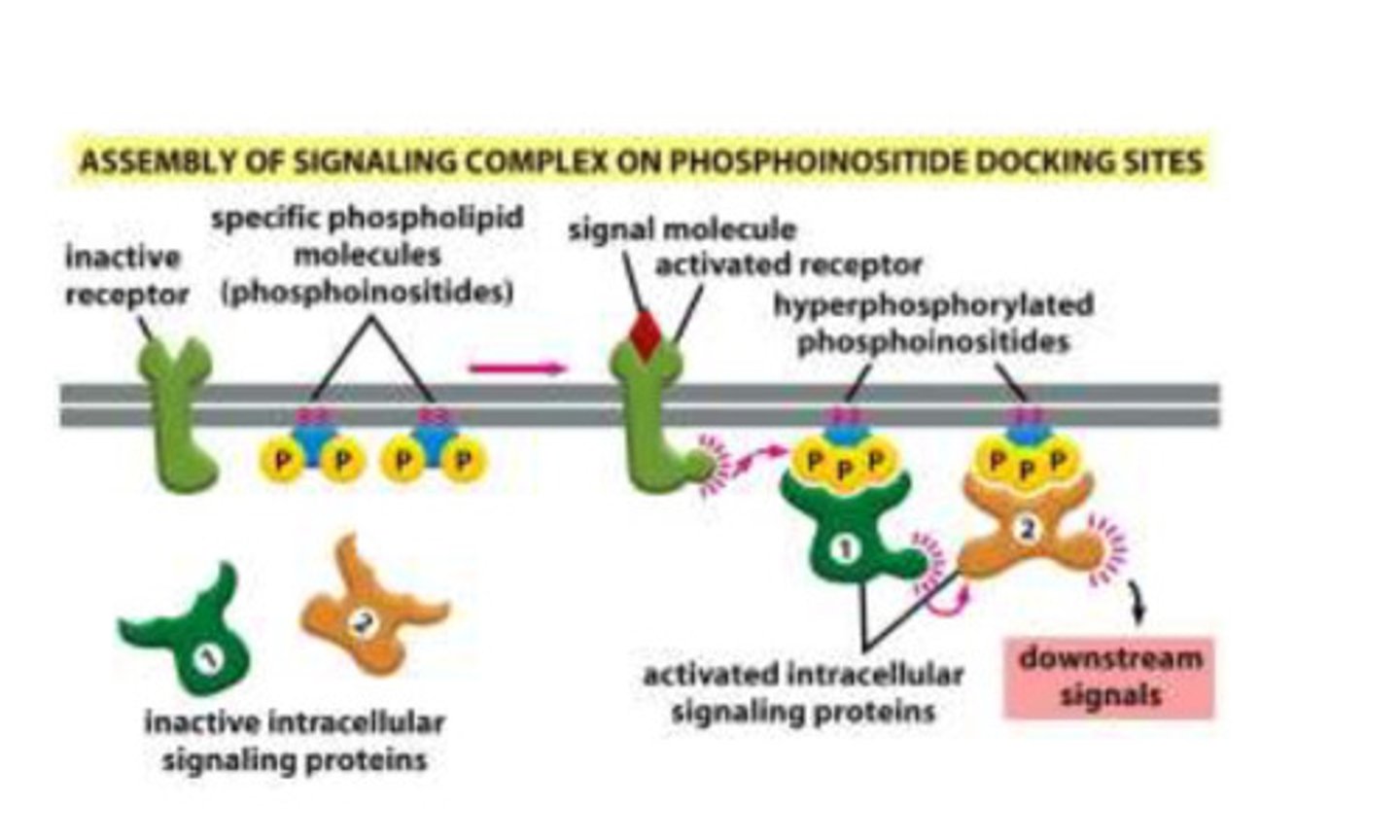
What is an example of a phosphoinositide signaling complex?
insulin
What are some methods to regulate how pathways respond to a signal?
- response timing
- sensitivity to the ligand
- range of the ligand
- persistence of the response
- signal processing (conversion of simple signal into a complex response)
- integration
- coordination
What is signal integration? What are the steps?
it is the process that requires two separate inputs to send a signal
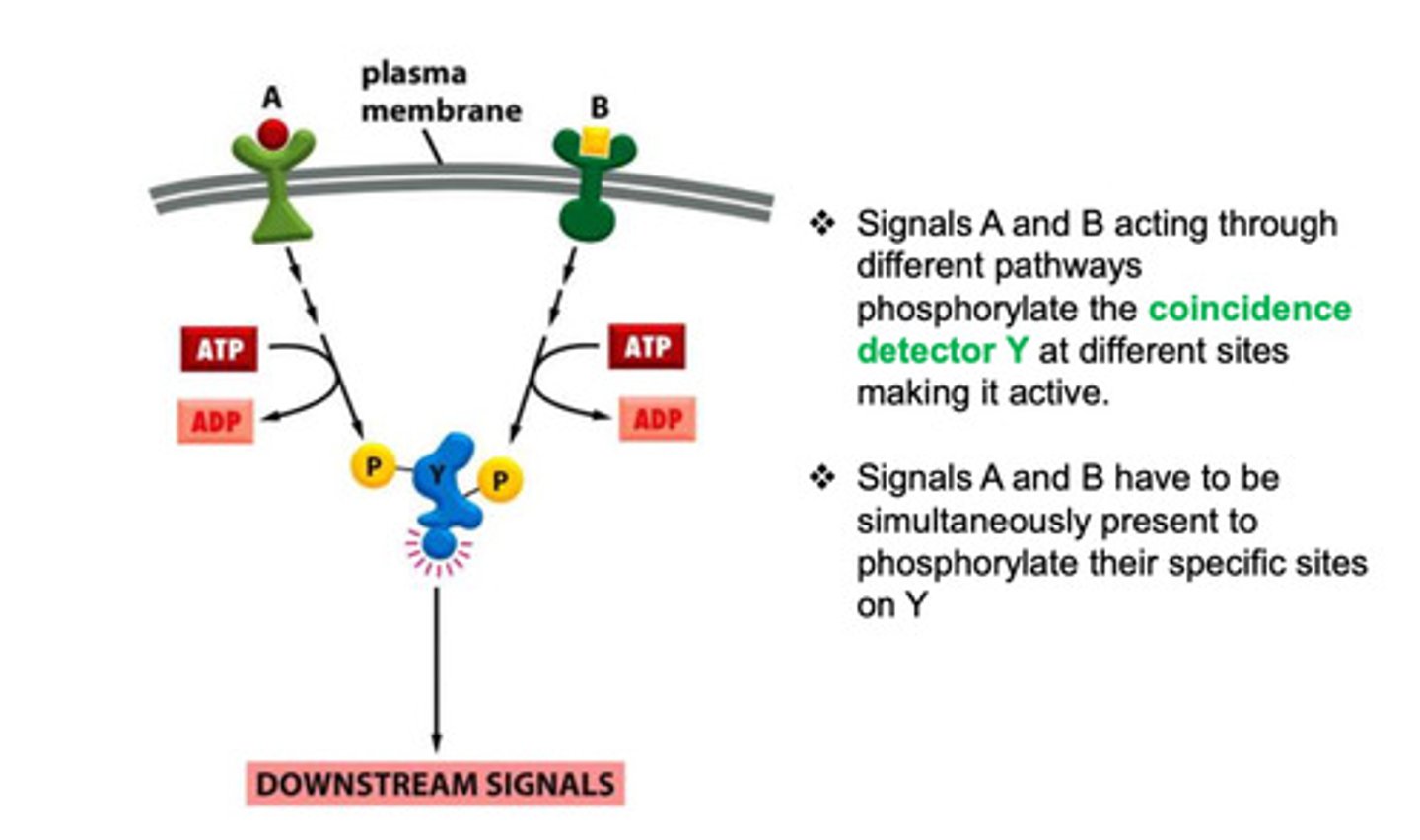
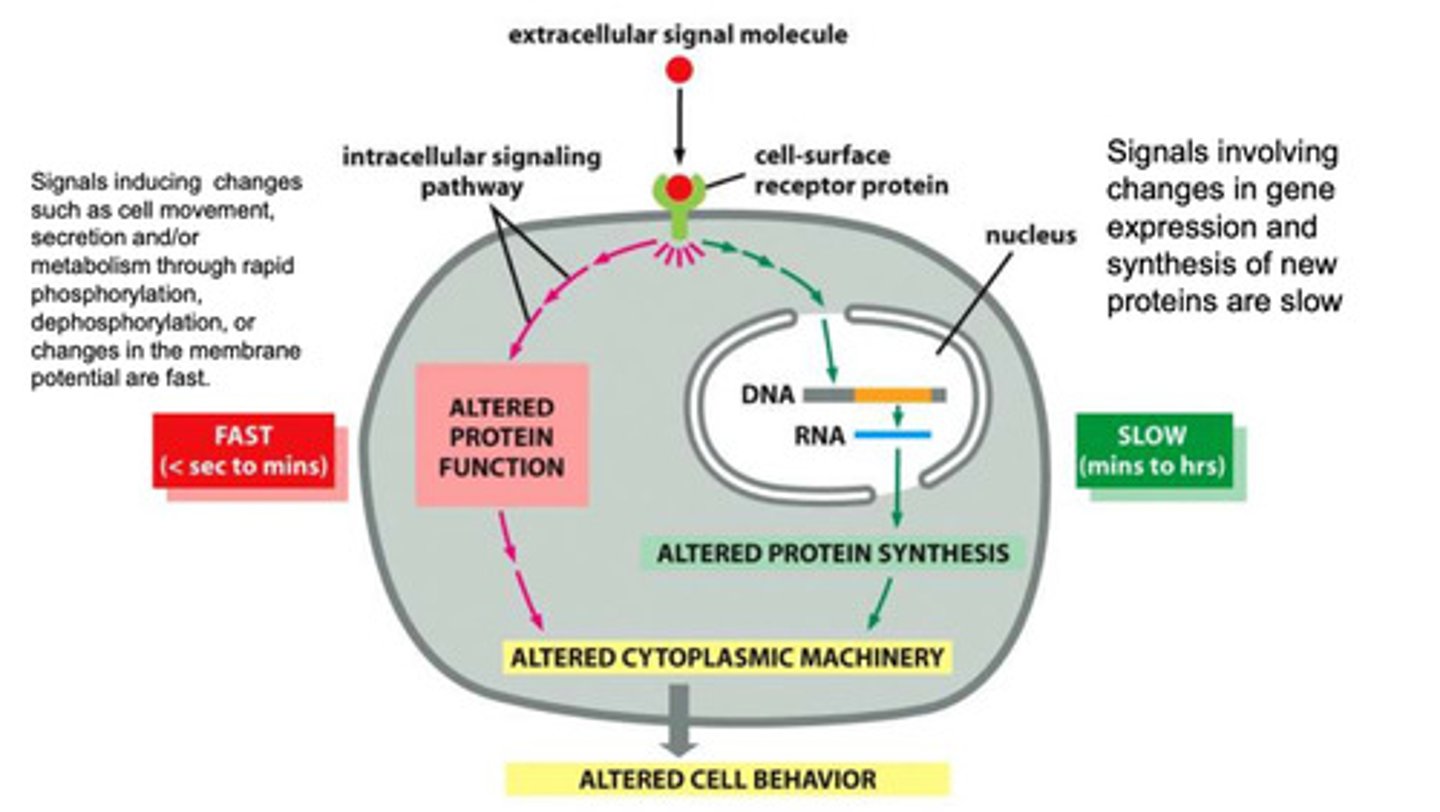
T/F Cell signals can cause both rapid and slow changes
True
fast: seconds to minutes
slow: minutes to hours
How are fast responses from extracellular signals typically carried out?
- the extracellular signal binds
- the signal is sent within the cell
- the target protein function is altered (via phosphorylation, dephosphorylation, or rapid membrane potential changes)
- the cytoplasmic machinery is altered by the protein, and the cell behavior changes
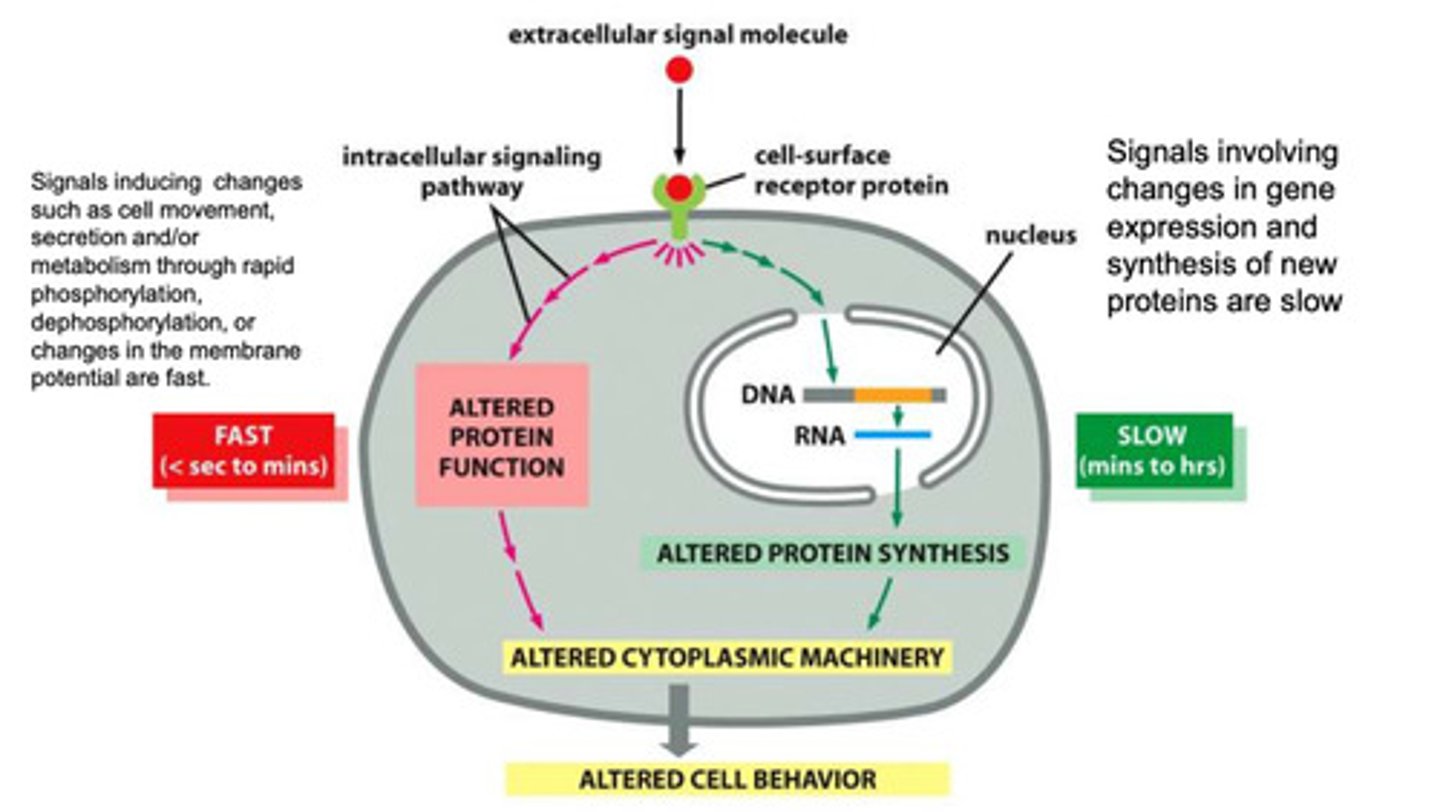
How are slow responses from extracellular signals typically carried out?
- the extracellular signal binds
- the signal is sent within the cell
- the signal is transmitted into the nucleus where is binds a specific protein to activate transcription of a specific mRNA
- the pretense of the protein alters the cytoplasmic machinery of the cell, and the cell behavior changes
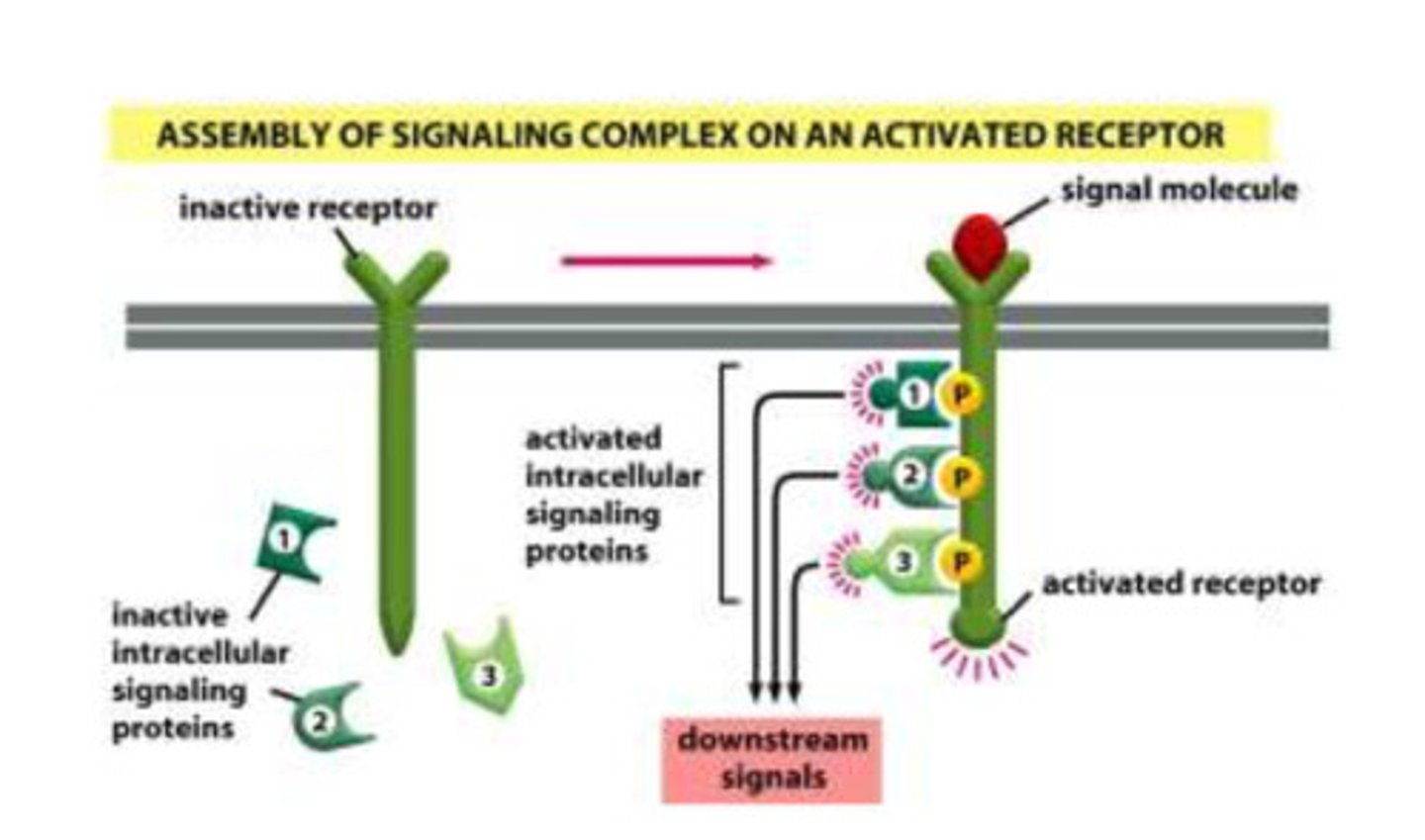
How do assembly signaling complexes transmit signals?
- the receptor starts off as inactive and the signaling proteins are found in the cytoplasm
- when the receptor is activated by the ligand, the receptor is phosphorylated
- when the receptor is phosphorylated, and the signaling proteins are recruited (unlike the pre-formed where the proteins are already present in the scaffold)
- the proteins then send the signal to the appropriate location so that the desired product can be made
What are feedback loops?
methods that cells utilize in order to have the product of an activated protein regulate the process in which the product is made
What are the types of feedback loops?
- positive feedback
- negative feedback
What is positive feedback and what are the steps?
- it is when the product of an activated protein stimulates the protein to make more of the product
- the protein is in an inactive state
- a signal is sent into the cell, and a protein is activated
- the activated protein then makes the product
- as long as the signal is present, the presence of the product stimulates the production of more of the product
- when the signal is removed, the product is no longer made, and the protein is turned off
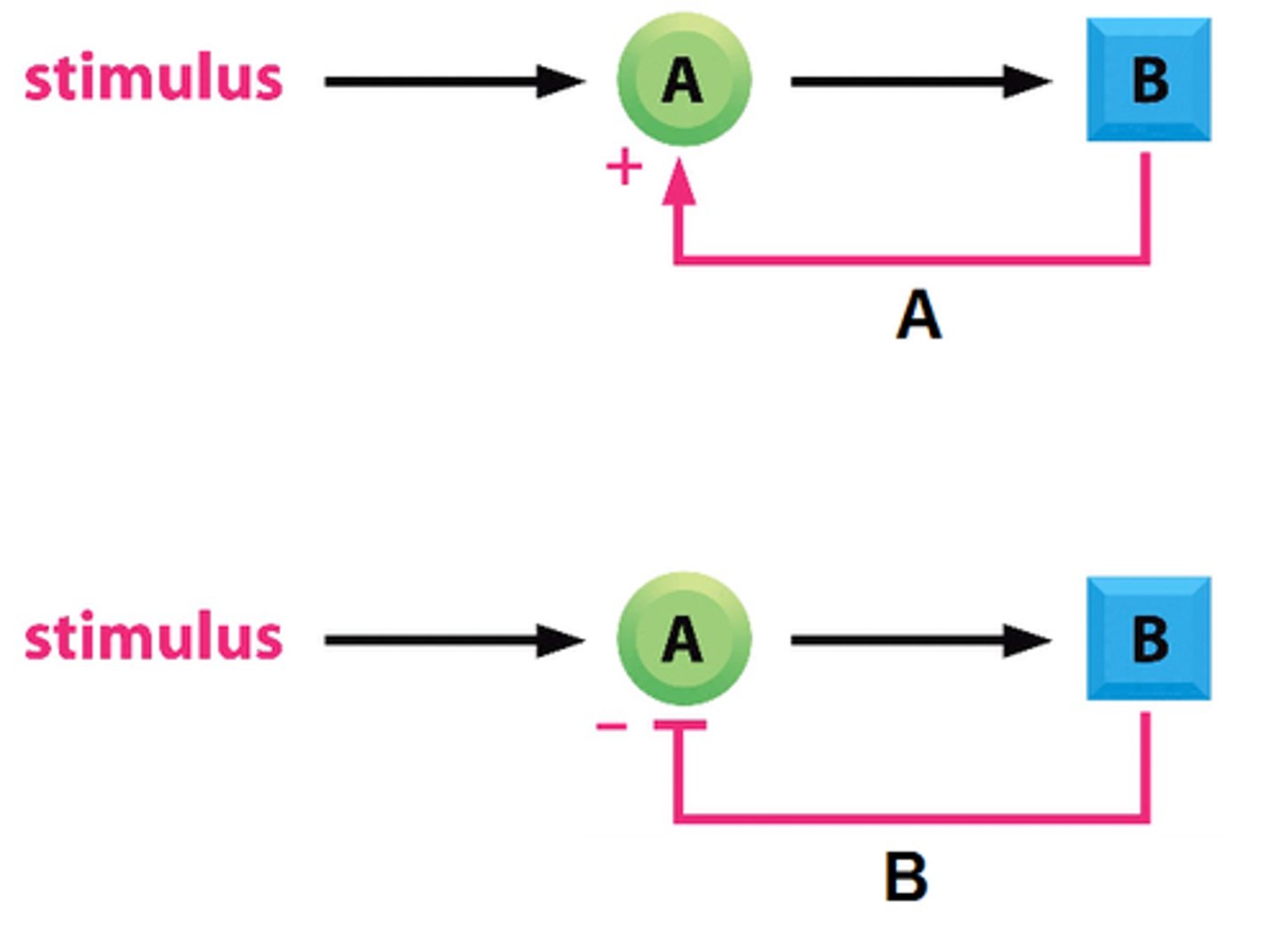
What is negative feedback and what are the steps?
- the protein is in an active state
- a signal is sent into the cell, and the products are degraded
- the degradation of the products causes the protein to be deactivated
- the deactivated protein THEN ceases to make any more product
- as long as the signal is present, the absence of the product inhibits the production of more product
- when the signal is removed, the product resumes being made, and the protein is turned back on
What are the types of cell surface receptors?
- ion channel-coupled receptors
- G-protein-coupled receptors
- enzyme-coupled receptors
What are the methods of adjusting sensitivity to an extracellular signal?
- receptor sequestration
- receptor down-regulation
- receptor inactivation
- inactivation of signaling protein
- production of inhibitory protein
How are receptors sequestered to prevent extracellular signaling?
the receptor and the signal are enveloped in an endosome, and separated
How are receptors down regulated to prevent extracellular signaling?
- the receptor/signal complex is enveloped
- lysosomes bind with the envelope and empty their contents into the envelope to degrade the receptor/signal complex
How are receptors inactivated to prevent extracellular signaling?
A inhibitory protein binds to the portion of the receptor protein that activates to send the signal
How are signals blocked via inhibition of signaling proteins?
- the signal binds to the receptor
- the receptor is activated, BUT the signal protein is inactivated, so the signal is unable to be transduced intracellularly
How does the production of inhibitory proteins inhibit extracellular signaling?
- the ligand binds to the receptor
- the receptor recruits the signaling protein
- the signal is transduced into the cell
- at some point, an inhibitory protein (that is already found in the cytoplasm or that has made to stop the specific reaction from occurring) binds, and that product is no longer made--regardless of if the ligand is still bound to the receptor or not
What is the method in which nitric oxide causes smooth muscle relaxation?
- acetylcholine binds to NO synthase (NOS)
- NOS activation leads the arginine being converted into nitric oxide
- the nitric oxide then diffuses across the membrane of the cell
- nitric oxide binds to guanylyl cyclase
- activation of guanylyl cyclase causes the production of cGMP
- cGMP then induces the rapid relaxation of smooth muscle cells
What is an ion-coupled receptor?
it is a receptor that allows for the flow of ions once the ligand protein binds
What are ion-couple receptors typically used for?
rapid synaptic signaling between electrically excitable target cells (like nerve/muscle cells)
G-protein coupled receptors are direct/indirect signal activators
- indirect
- the act by mediating the interactions between the activated receptor and the target protein
What are the largest family of cell surface receptors?
G-proteins
T/F G protein structure differs significantly for each type fo receptor
false, they have similar structures
T/F Because g-proteins have similar structures, a single g-protein ligand can activate many different g-protein coupled receptors
true. even though the receptors all have the same ligand, they all have different responses because they are found in difference cells
how many units a G-proteins composed of?
3: α, β, and γ
What is the structure of an inactive G-protein?
- there is no ligand present on the extracellular surface receptor
- there is a GDP bound to the α subunit, and the βγ subunit complex is bound to the α subunit
What occurs when a stimulatory cAMP G-protein is activated? What is a toxin that utilizes this method?
- a ligand binds to the extracellular surface receptor of the G-protein
- the binding of the ligand activates the G-protein
- when the protein is activated, the GDP is exchanged for GTP (via GEF)
- the presence of GTP causes the α subunit and the βγ subunit complex to dissociate from one another
- the activation causes adenylyl cyclase to produce high levels of cAMP
- cAMP then binds to the regulatory units bound to PKA
- the binding of cAMP to the regulatory units releases and activates PKA
- activated PKA then goes into the nucleus
- activated PKA phosphorylates CREB
- the phosphorylated CREB binds to CBP
- the CREB/CBP complex then binds to the cyclic AMP response element on the DNA strand
- the target gene is then transcribed and produced
- Cholera

What occurs when an inhibitory cAMP G-protein is activated? What is a toxin that utilizes this method?
- a ligand binds to the extracellular surface receptor of the G-protein
- binding of the g-protein causes GTP to be dephosphorylated into GDP
- the presence of GDP causes the α subunit and the βγ subunit complex to reassociate
- the reassociation of the subunits inhibits adenylyl cyclase
- the deactivation causes adenylyl cyclase prevents the production of cAMP
- cAMP is degraded by cAMP phosphodiesterase
- cAMP is no longer available to bind to the regulatory proteins of PKA, so the two proteins reassociated
- reassociation deactivates PKA and the product is no longer made
- Pertussis
What are the types of PKA?
- type I
- type II
Where are type I PKA molecules found?
in the cytoplasm
Where are type II PKA molecules found?
in membranes (plasma, nuclear, mitochondrial) and microtubules
What are two senses that rely on G-protein channel receptors?
- smell
- vision
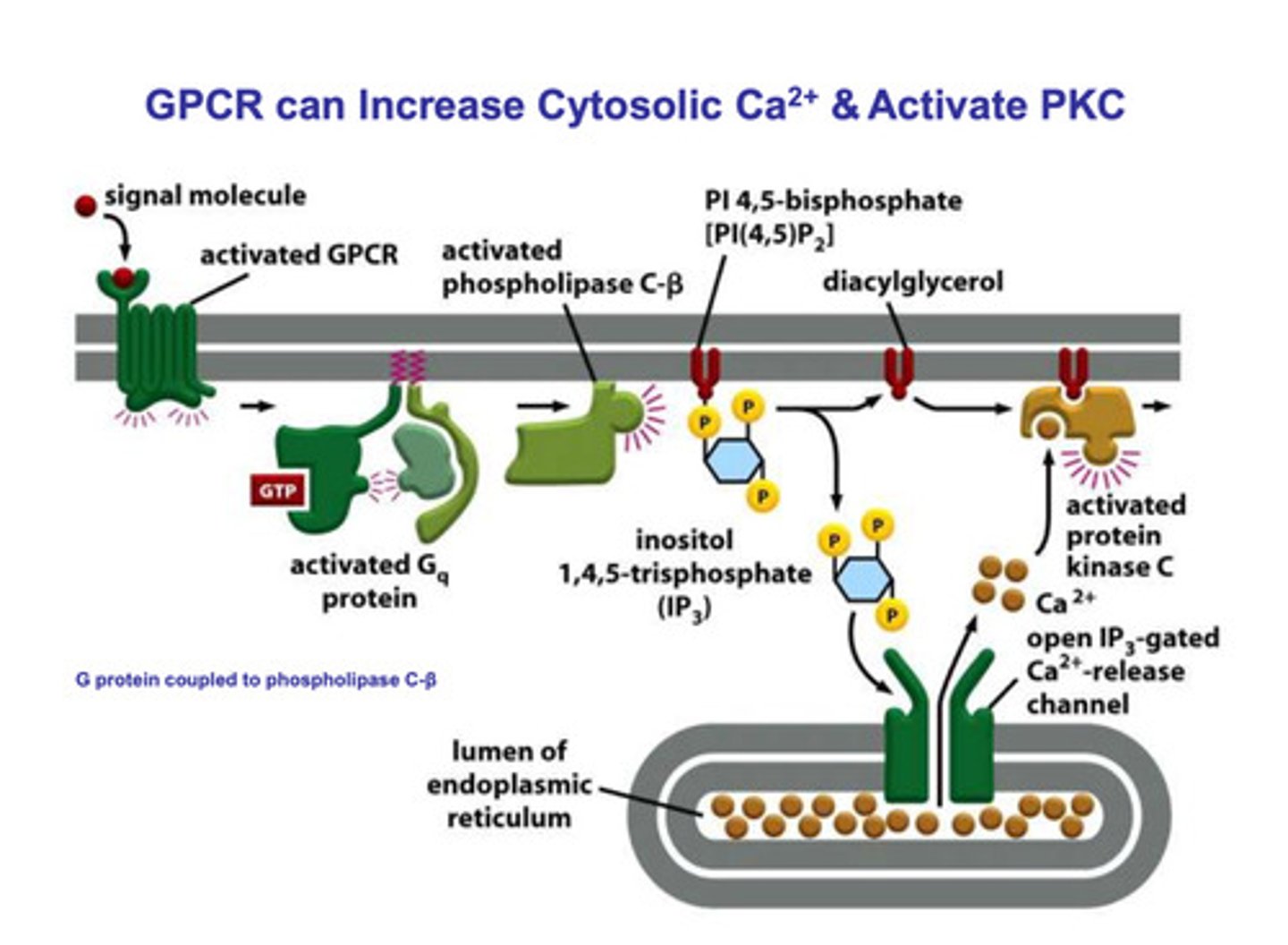
How do G-proteins activate PKC?
- via calcium release
- a signal protein binds to the receptor of the G-protein
- the α subunit and the βγ subunit complex dissociate
- the dissociation of the α subunit and the βγ subunit complex activates phospholipase C-β
- Phospholipase C-β phosphorylates IP3, which causes it to dissociate from diacyglycerol
- IP3 binds to a IP3 gated channel on the endoplasmic reticulum and an inactive PKC binds to the diacylglcerol
- the binding of IP3 opens the calcium channels, and the calcium flows out of the endoplasmic reticulum, and into the cell
- calcium binds to the PKC/diacylglycerol complex and activates PKC to produce the response for the signal
High/low levels of calcium are desired in the cytosol. How are those levels maintained?
- low
- Na+/Ca2+ exchanger (Ca2+ out)
- Ca2+ pump (Ca2+ out)
- Ca2+ pump on endoplasmic reticulum (Ca2+ in)
- Ca2+ binding molecules in the cytoplasm
- Ca2+ import molecules in mitochondria (also pump in H+)
How do G-proteins relay a visual signal to the brain?
- a rhodopsin molecule absorbs a photon
- activates G-proteins (ex. 500)
- cGMP phosphodiesterase activated (500)
- cGMP molecules are hydrolyzed (10^5)
- cation channels close (250)
- membrane potential changes
- message sent to brain
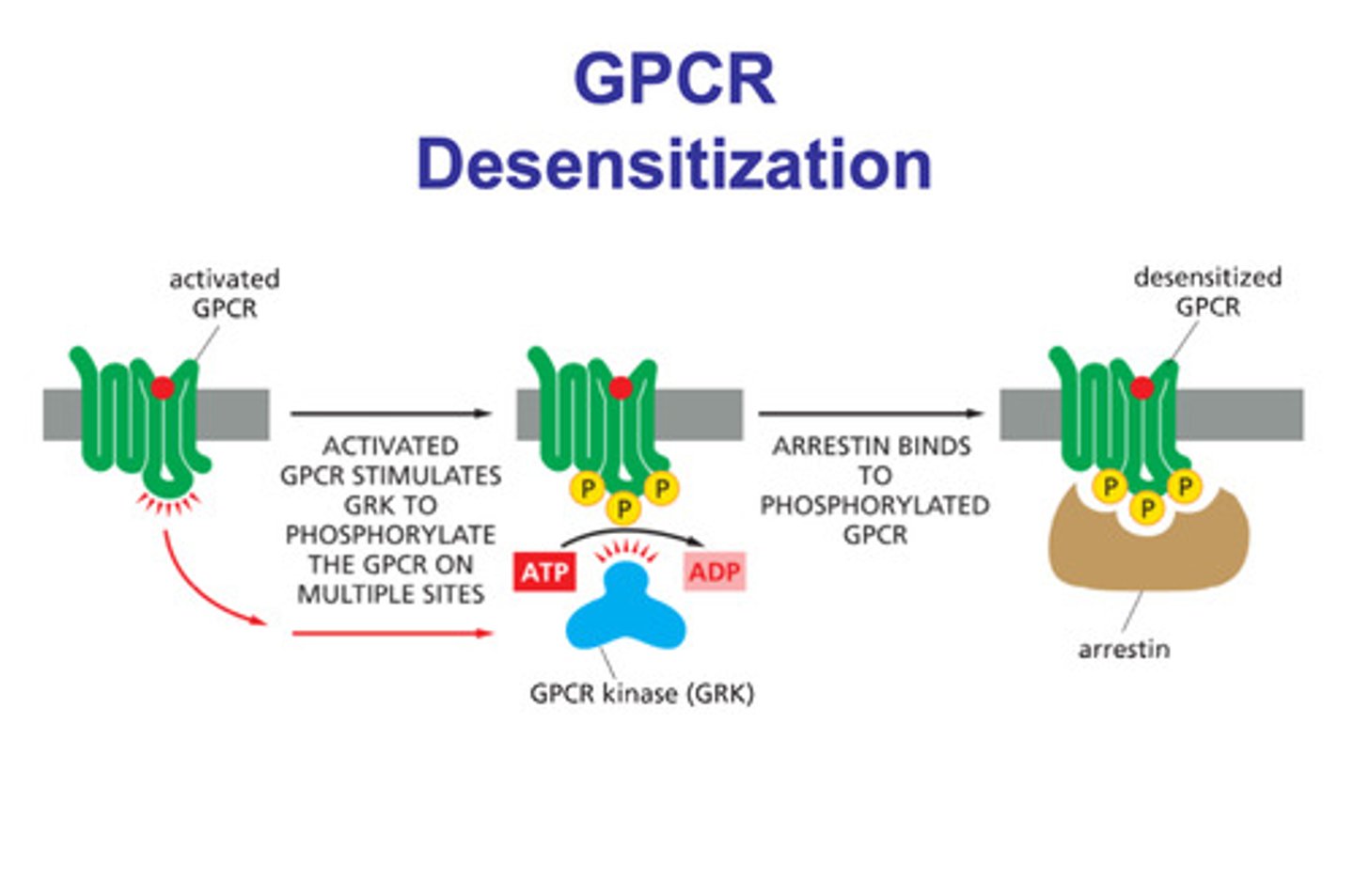
How are G-protein receptors desensitized? what are the steps?
- arrestin molecules
- a ligand binds to a g-protein receptor
- GPCR kinase phosphorylates the activated g-protein (phosphate groups)
- arrestin binds to the phosphorylated g-protein (with the ligand stilll bound) and the protein is desensitized
How do enzyme-coupled receptors work?
the receptor acts as an enzyme OR is associate directly WITH enzymes that activate the receptor
Where are enzyme-coupled ligand binding sites located? catalytic binding site or enzyme binding site?
- on the outer surface of the cell
- on the inner surface of the cell
What are the most prevalent kind of enzyme coupled receptor?
receptor tyrosine kinases
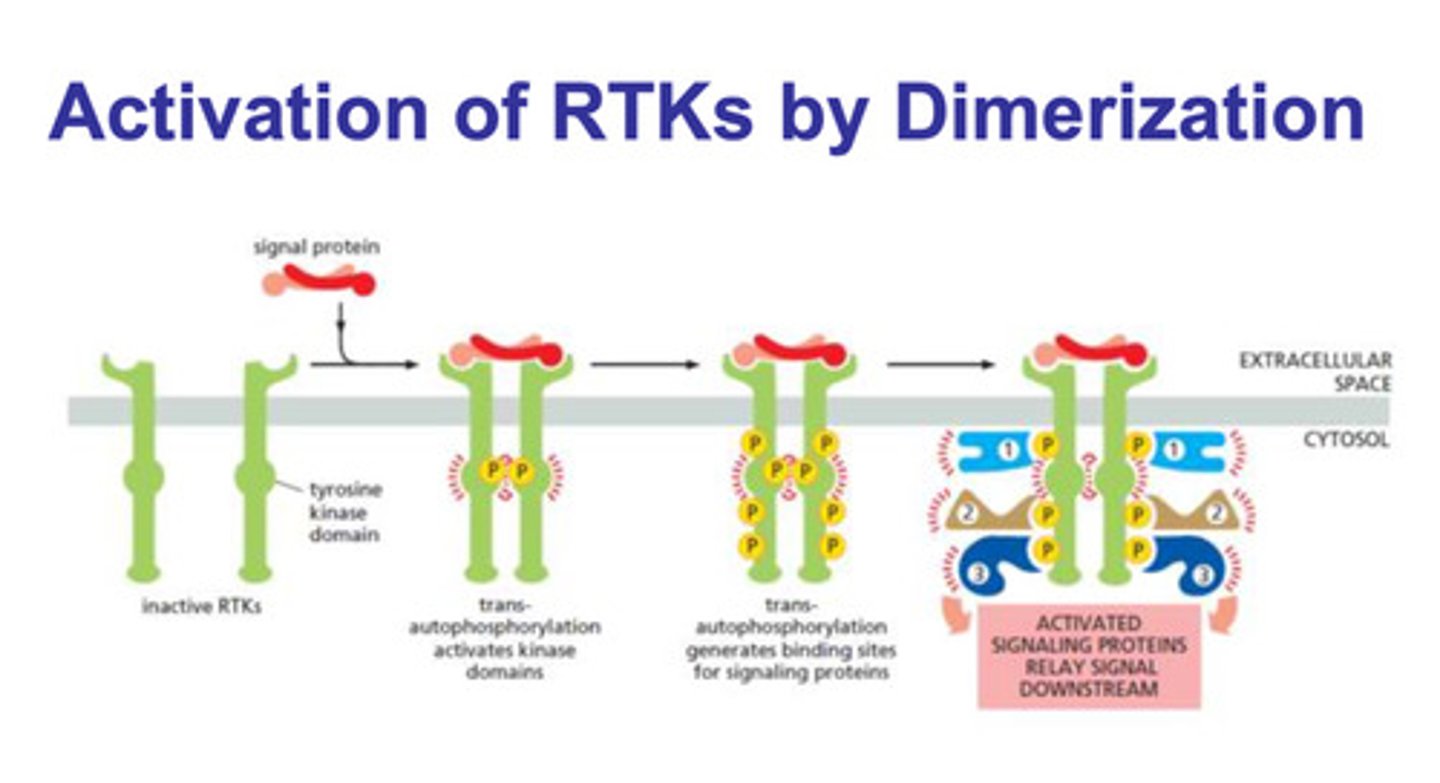
How are receptor tyrosine kinases (TRK) activated? what are the steps?
- dimerization
- the two parts of the TRK domain are separated and inactive
- a signal protein binds to the both of them, and they undergo trans-autophosphorylation (also called cross-autophosphorylation)
- the phosphorylation of the domain generates binding sites for the signaling proteins
- the signaling proteins bind, and the signal is relayed downstream
How do tyrosine kinase receptors activate PKC?
- the two parts of the TRK domain are separated and inactive
- a signal protein binds to the both of them, and they undergo trans-autophosphorylation (also called cross-autophosphorylation)
- the phosphorylation of the domain generates binding sites for PI3 kinase
- IP3 kinase phosphorylates IP3 (but the IP3 remains attached to the diacylglycerol)
- the signaling protein binds, and the signal is transduced into the cell
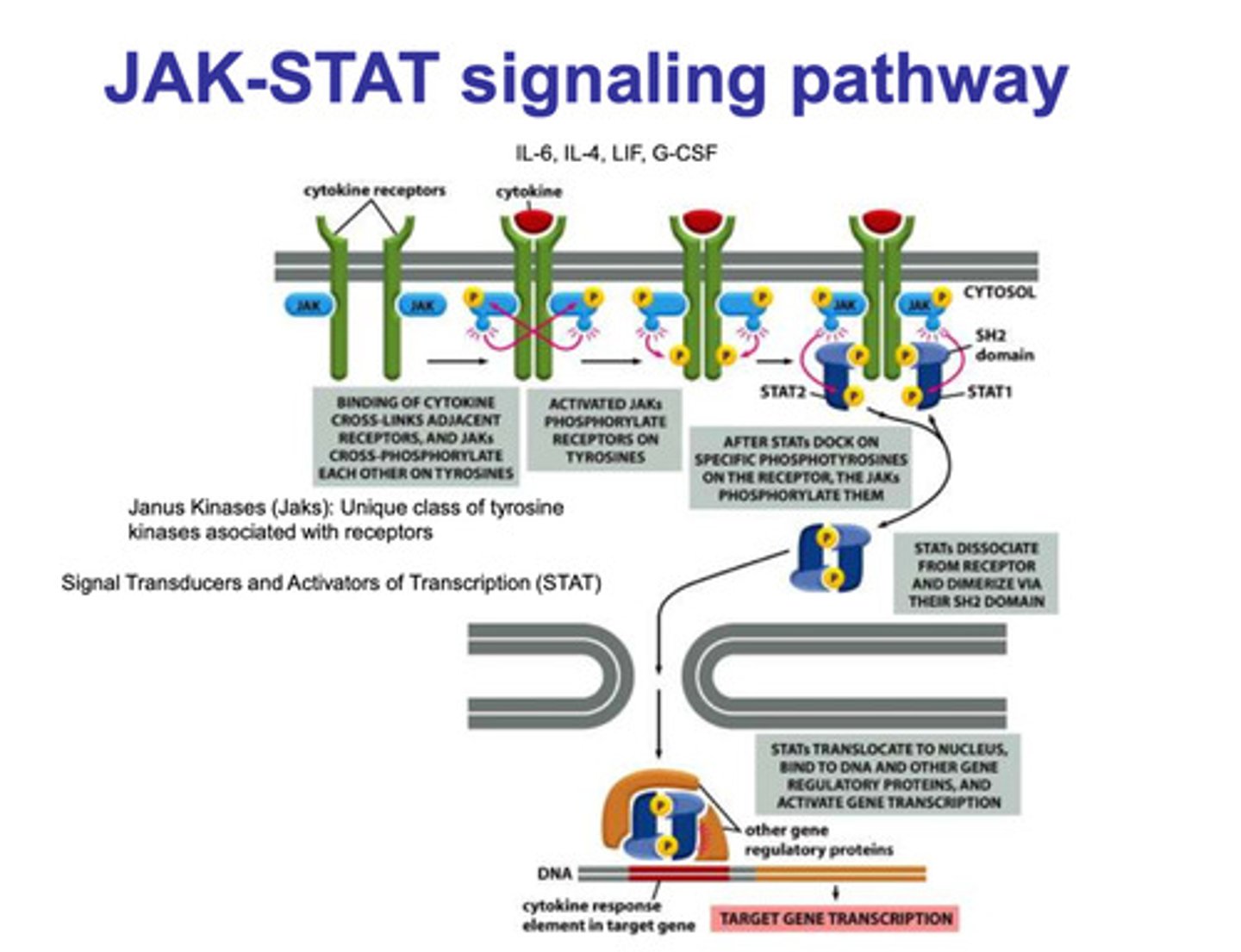
What is the JAK-STAT signaling pathway?
- two cytokine receptors with JAK proteins attached are inactive
- a cytokine binds to the two receptors which causes the JAK proteins to cross-phosphorylate each other an become activated
- activation of the JAK proteins causes phosphorylation of the tyrosine receptor
- the phosphorylation of the tyrosine creates binding areas, and STAT 1 and STAT2 bind
- JAK then phosphorylates both proteins
- STAT1 and STAT2 dissociate from the tyrosine receptor and dimerize
- the dimerized STAT proteins are then transported into the nucleus
- other regulatory proteins bind to the dimer, and they complex binds to the cytokine response element in the target gene
- gene transcription is then upregulated
What are JAK-STAT pathways important for?
- medications
- they can be used to turn down immune response (important for transplant rejection)
- can be useful with treatment for rheumatoid arthritis
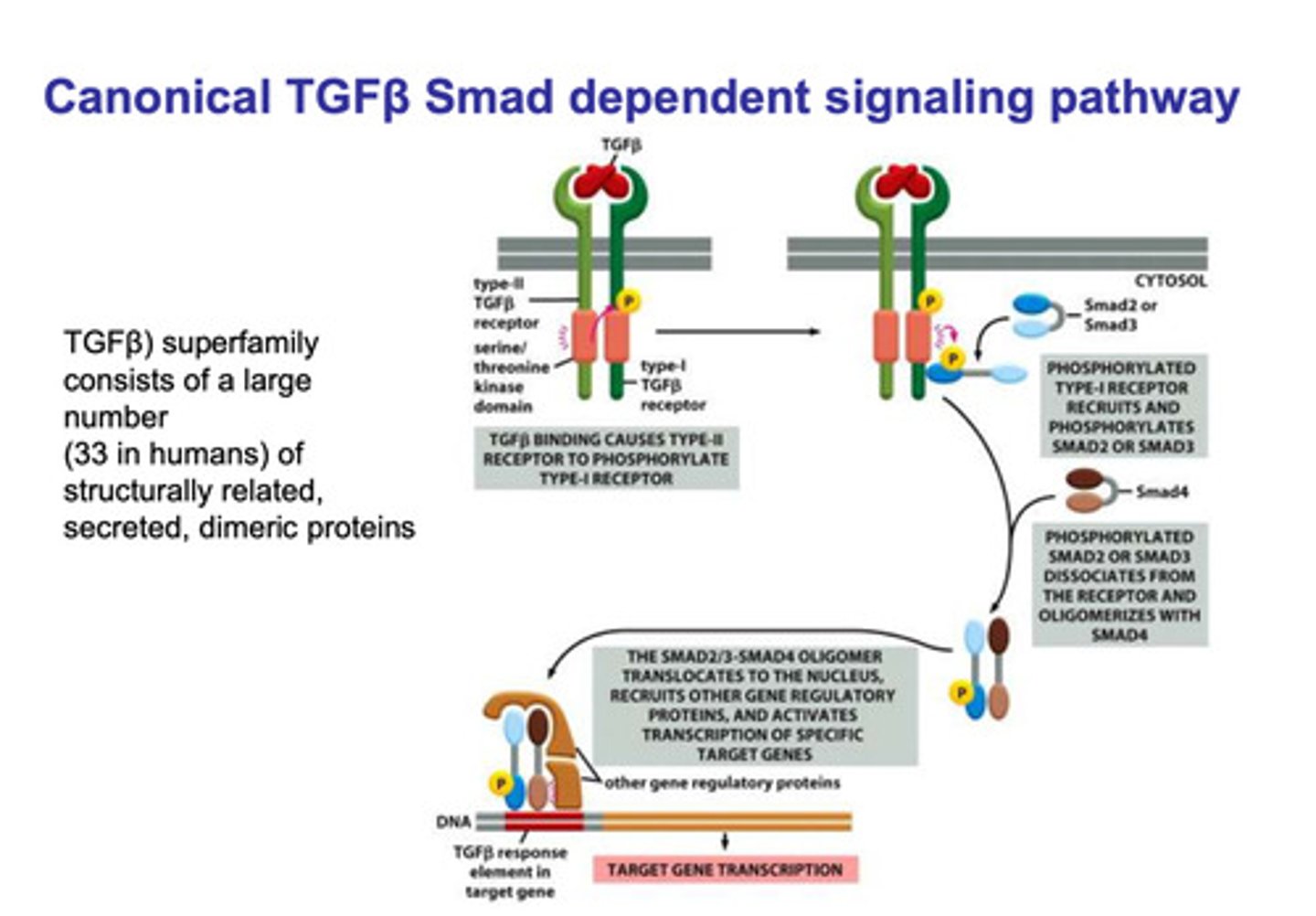
Canonical TGFβ SMAD dependent signaling pathway
- TGFβ binds to the type II and type I TGFβ cytokine receptors
- a serine/threonine kinase on the the type II domain phosphorylates the type I domain
- phosphorylation of the type I domain recruits SMAD2 or SMAD3
- the SMAD molecule is the phosphorylated, which causes it to dissociate and straighten
- the straightened SMAD2 or SMAD3 oligomerizes with SMAD4
- the oligomer is transported into the nucleus where is binds with other regulatory molecules
- the complex binds to the TGFβ response element in the target gene
- transcription of the product
SMAD fibrosis signaling pathway
- TGF-β1 cytokine binds to two domain proteins (TβRII and TβRI)
- the two proteins are phosphorylated, which recruits SMAD2 and SMAD3 to bind to TβRI
- SMAD4 is recruited by the SMAD2/SMAD3 complex, and dissociates from TβRI
- the SMAD2/SMAD3/SMAD4 complex binds to a response element on the DNA strand
- fibrosis is induced
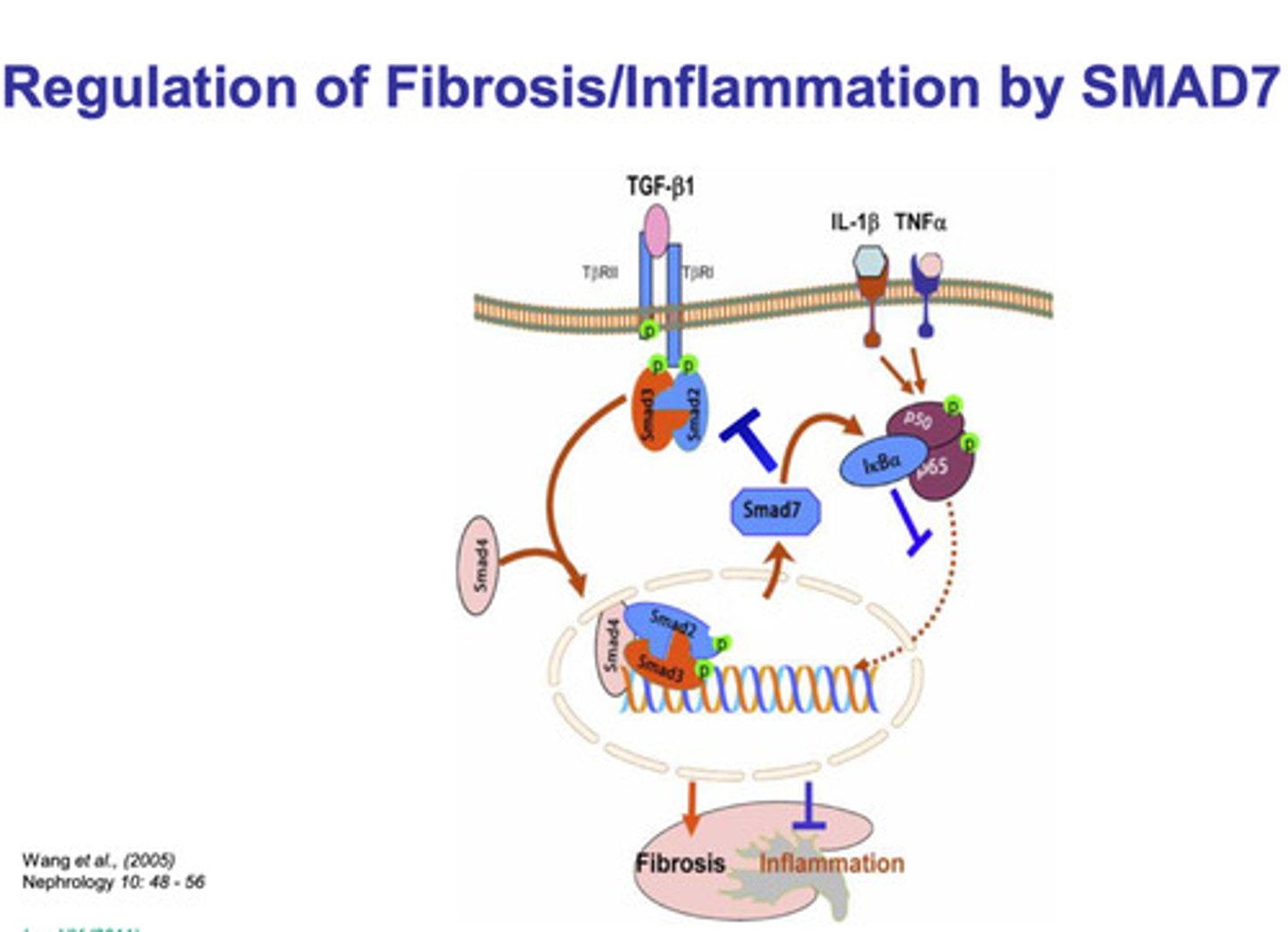
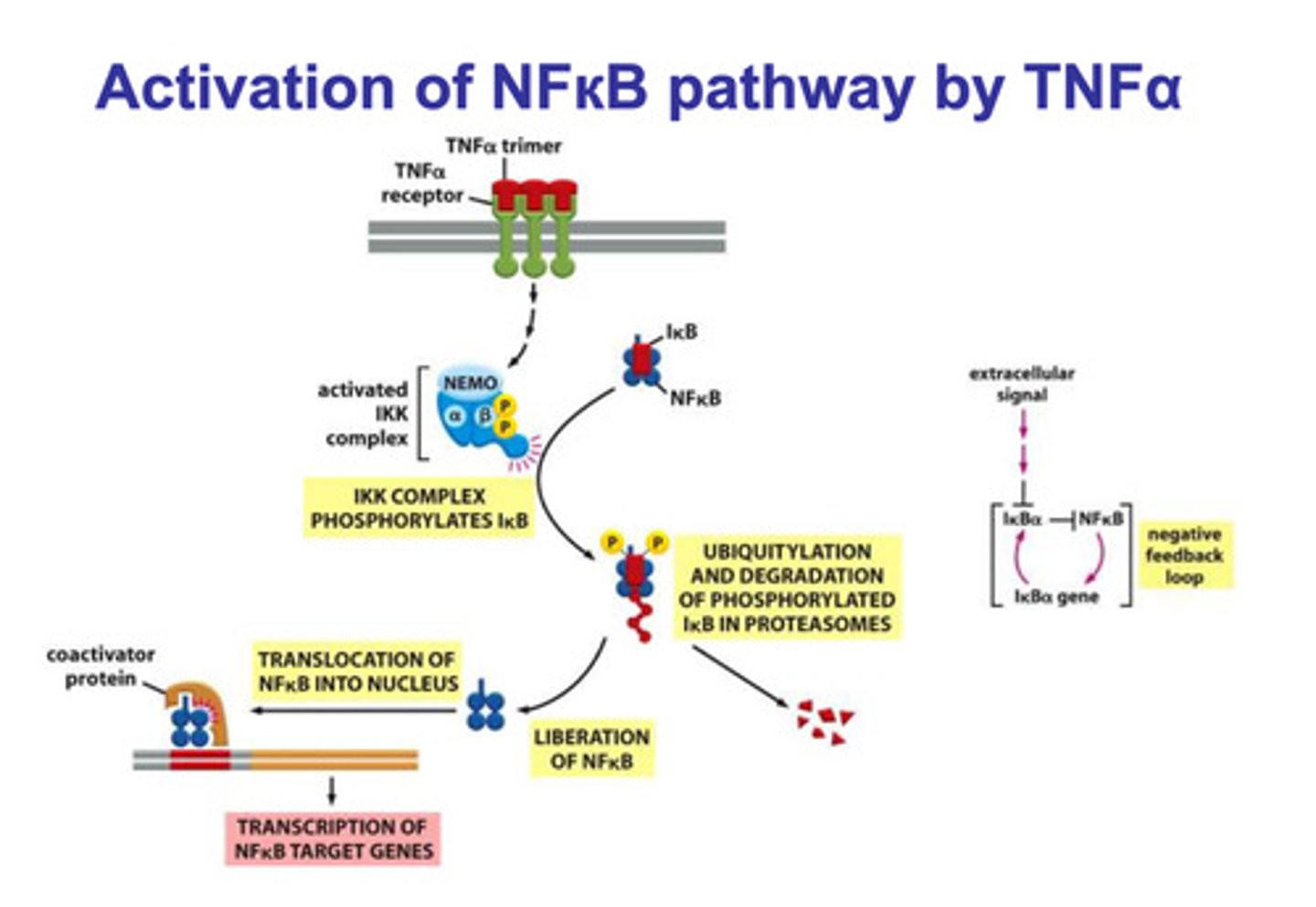
NFκβ signaling pathway via TNFα
- a TNFα trimer ligand binds to a TNFα trimer receptor
- once bound, the IKK complex is activated
- the activated IKK complex phosphorylates Iκβ
- the phosphorylation of Iκβ targets it for ubiquitination and degradation
- the degradation of Iκβ releases NFκβ, and it is translocated into the nucleus
- NFκβ and some additional regulatory proteins bind to the response element of the DNA strand
- transcription of NFκβ target genes are produced (pro-inflammatory genes)
(the production of NFκβ causes the production of Iκβ, which inhibits NFκβ)
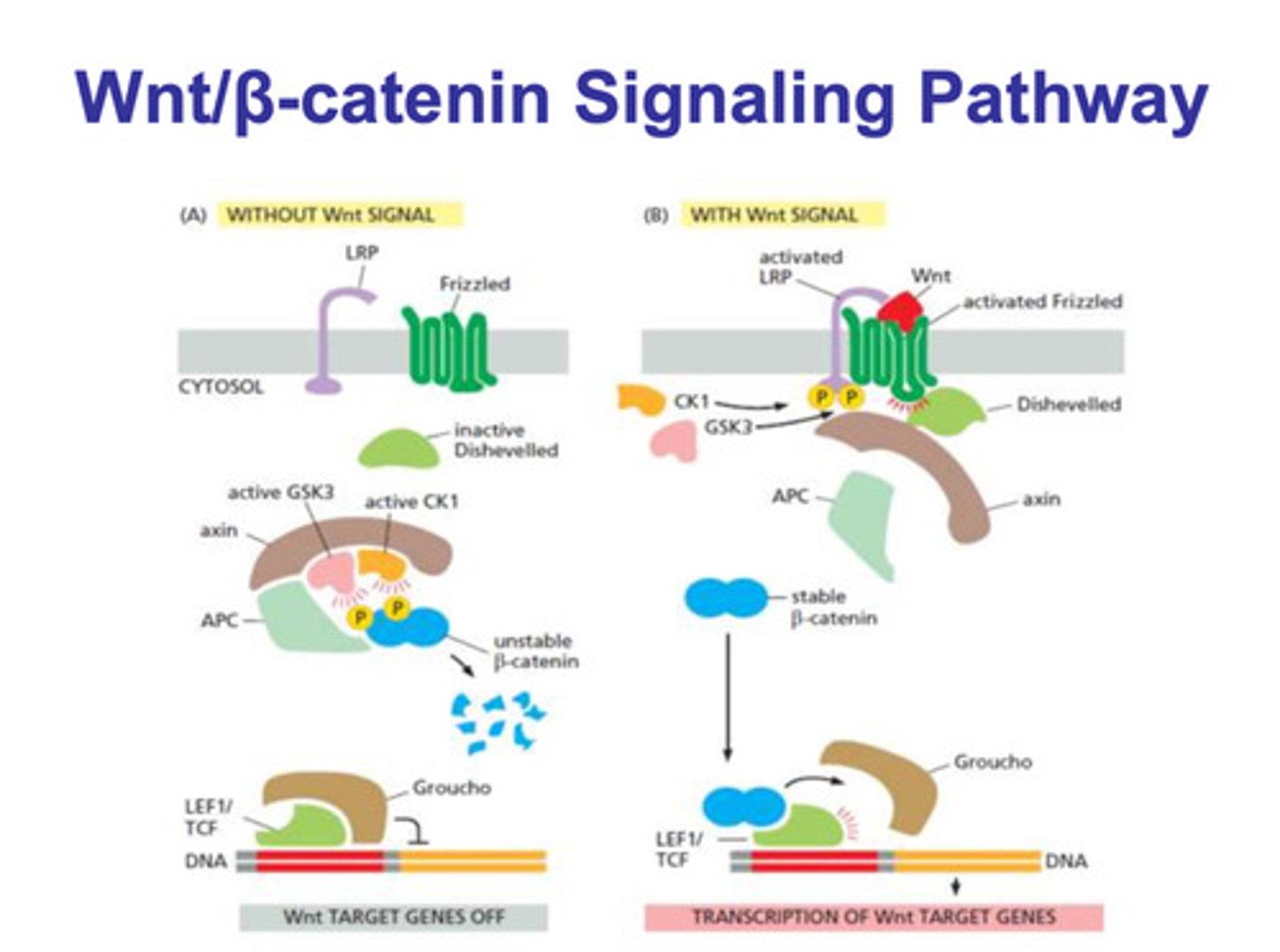
Wnt/β-catenin without a signal
- LRP and frizzled are dissociated
- dishevelled is inactivated
- APC, axin, CK1, GSK3 are associated in a complex
- CK1 and GSK3 phosphorylate β-catenin
- the phosphorylation of β-catenin targets the molecule for degradation
- Groucho remains bound to LEF1/TCF and gene transcription is turned off
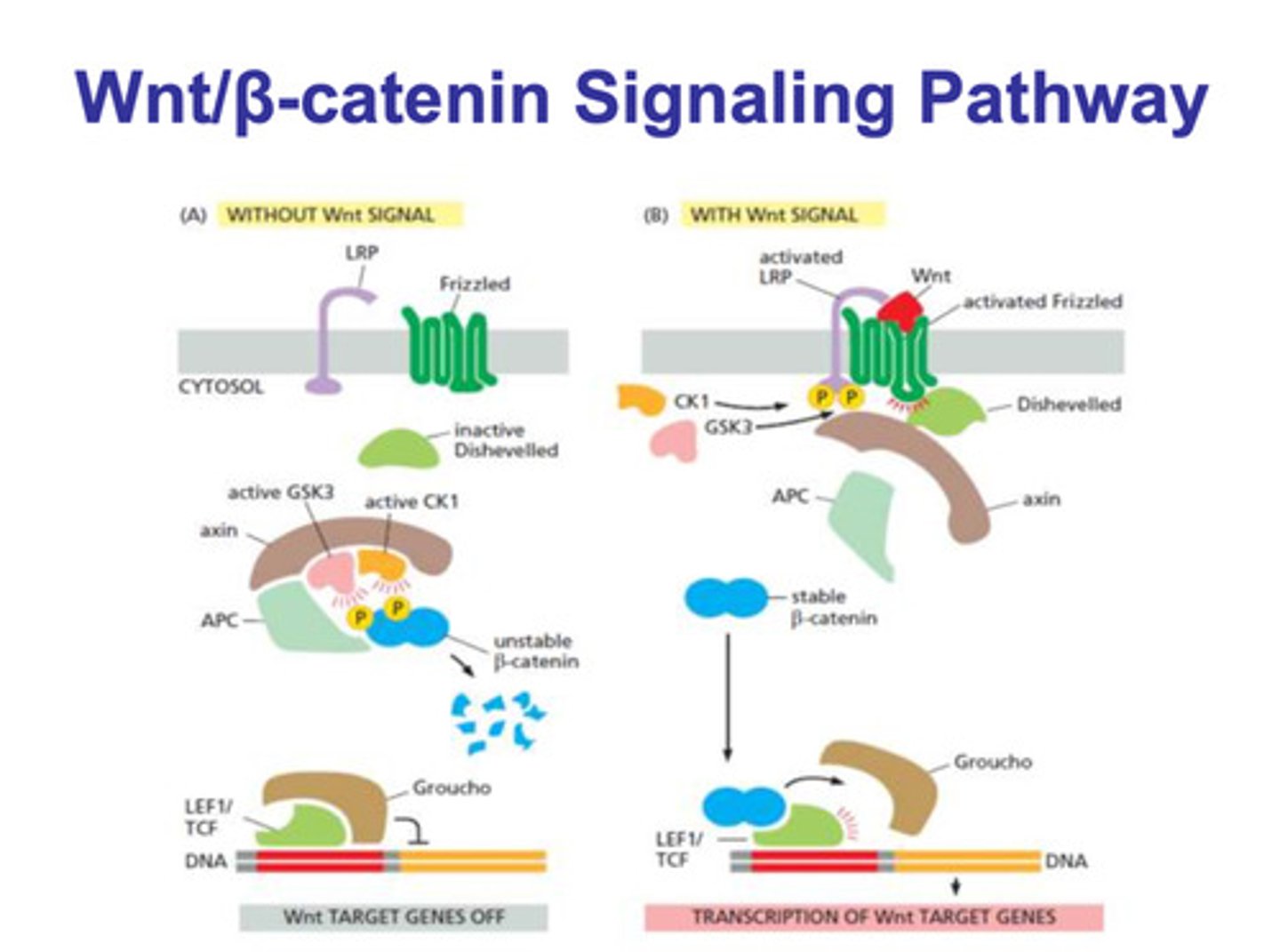
Wnt/β-catenin WITH a signal
- WNT binds to frizzled and LRP, which activates it both of them
- the activation of frizzled activates dishevelled
- CK1 and GCK3 phosphorylate LRP
- axin associates with the phosphorylated LRP and dissociates from APC
- because the APC/axin/GSK3/CK1 complex isn't formed, the β-catenin molecule is left alone
- β-catenin binds to the LEF1/TCF molecule, and Groucho dissociates
- the dissociation of Groucho allows for transcription of the target genes
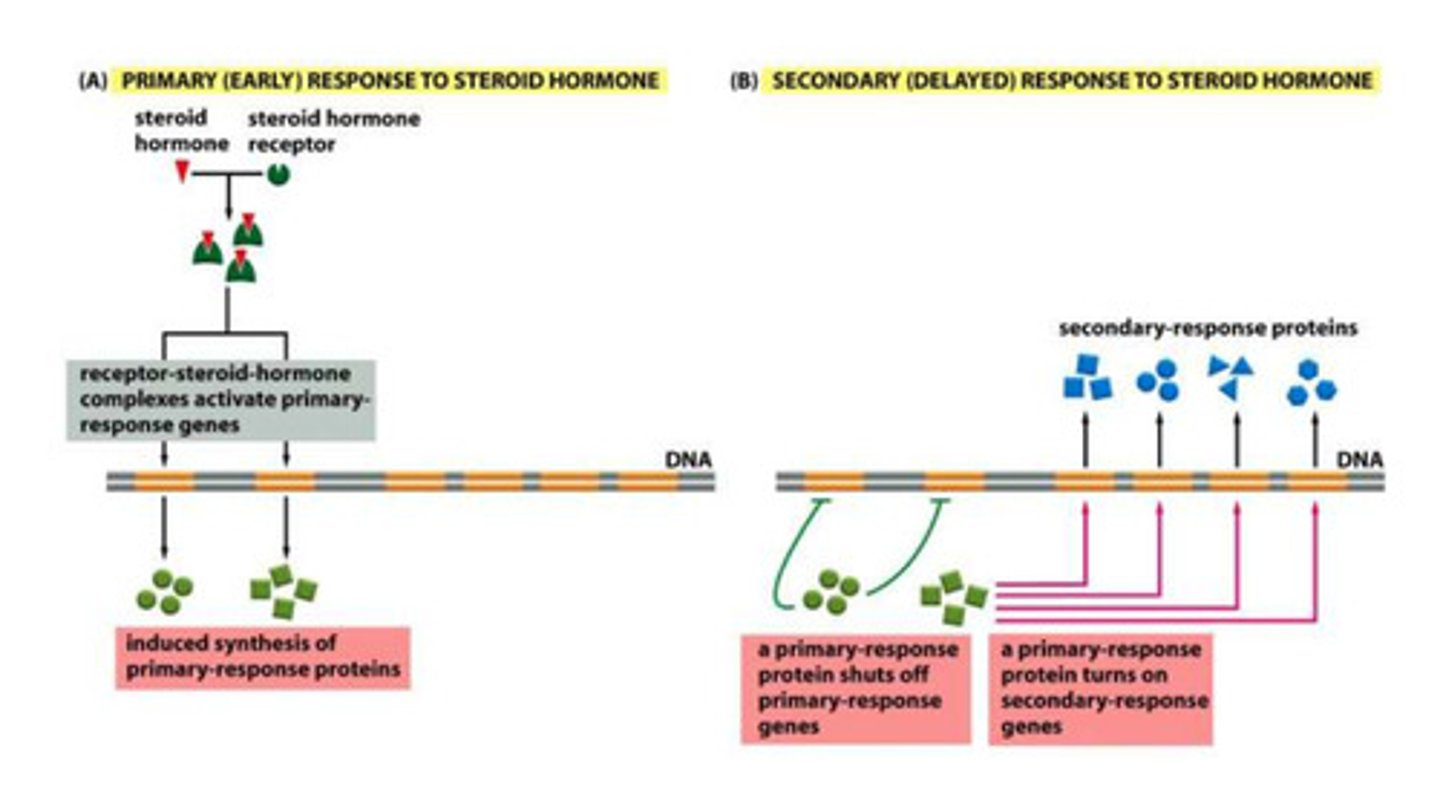
Primary hormone response (early)
- steroid hormones bind to the steroid receptors
- the complex then binds to the response sequences to transcribe the primary response genes
- the primary response protein are the produced
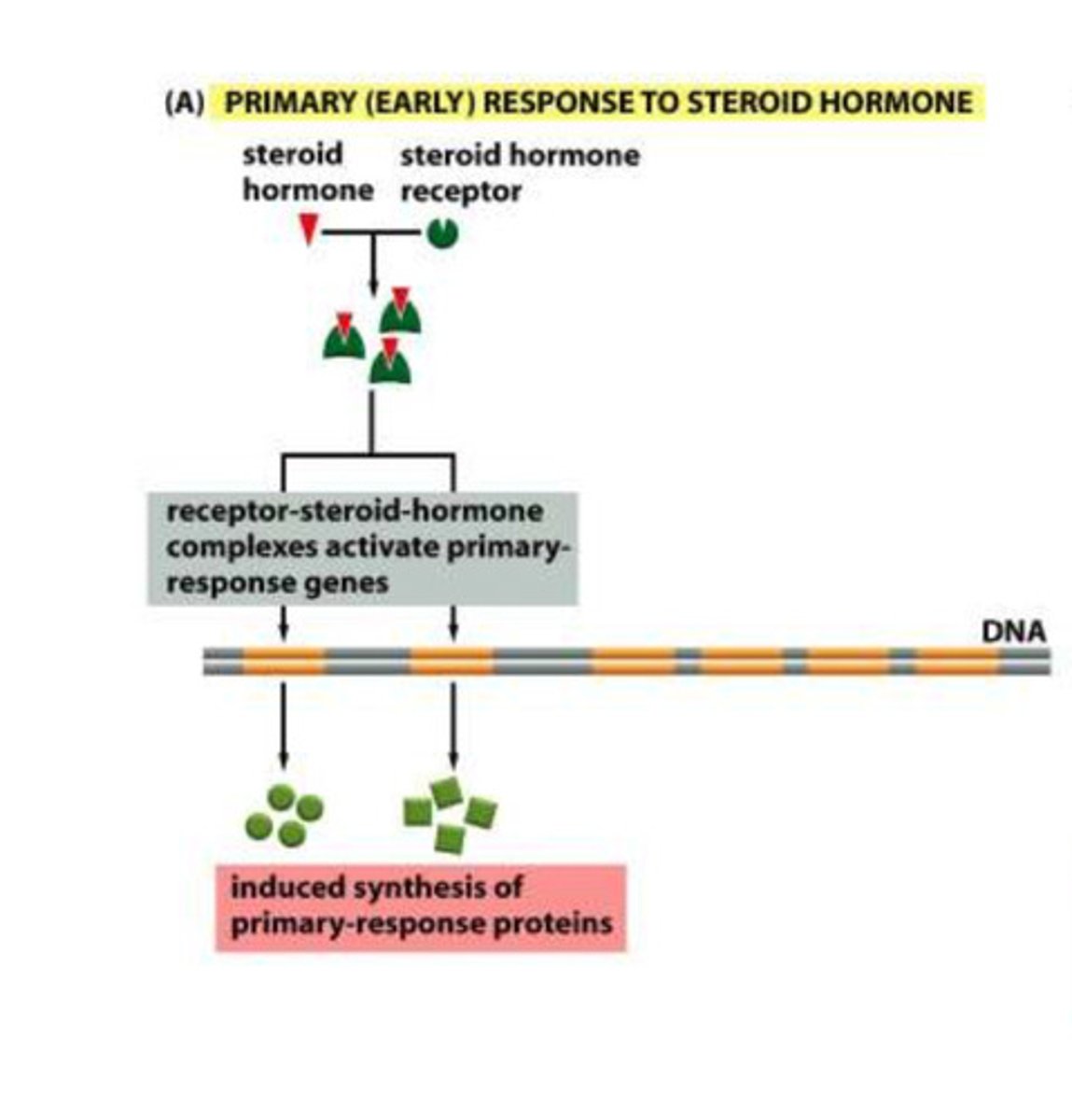
Secondary hormone response (delayed)
- the production of primary proteins is turned off BY the primary response protein product
- the primary proteins then bind to the primary response gene sequence to transcribe secondary proteins
- the secondary proteins are then produced