CH. 8 | Atoms and Periodic Properties
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Electron’s charge-to-mass ratio

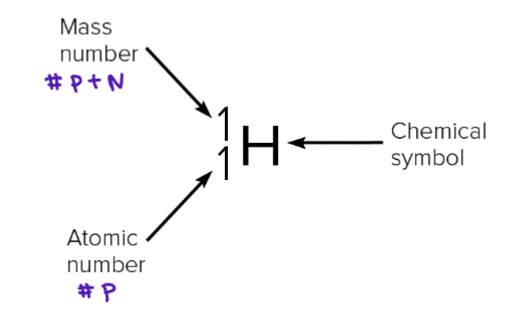
Atomic Number
# of protons in the nucleus
Elements distinguished by atomic #
# of protons = # of electrons in neutral atoms
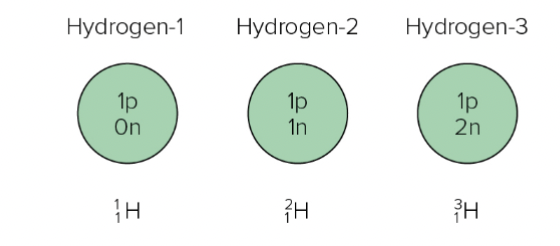
Isotopes
Same # of protons and electrons, different # of neutrons
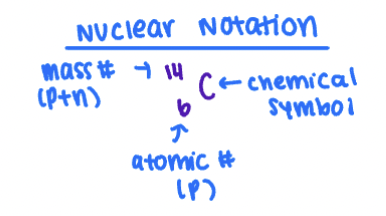
Nuclear Notation
Periodic Table Notation
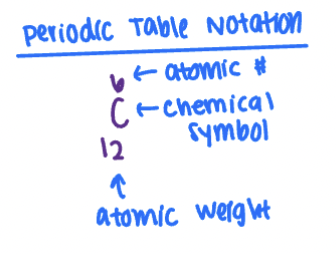
Mass Number
Number of protons + neutrons
Atomic Mass Unit
1/12 of carbon - 12 isotope mass
Atomic Weight
Atomic mass of an element, averaged over naturally occurring isotopes
Quantum Mechanics
…
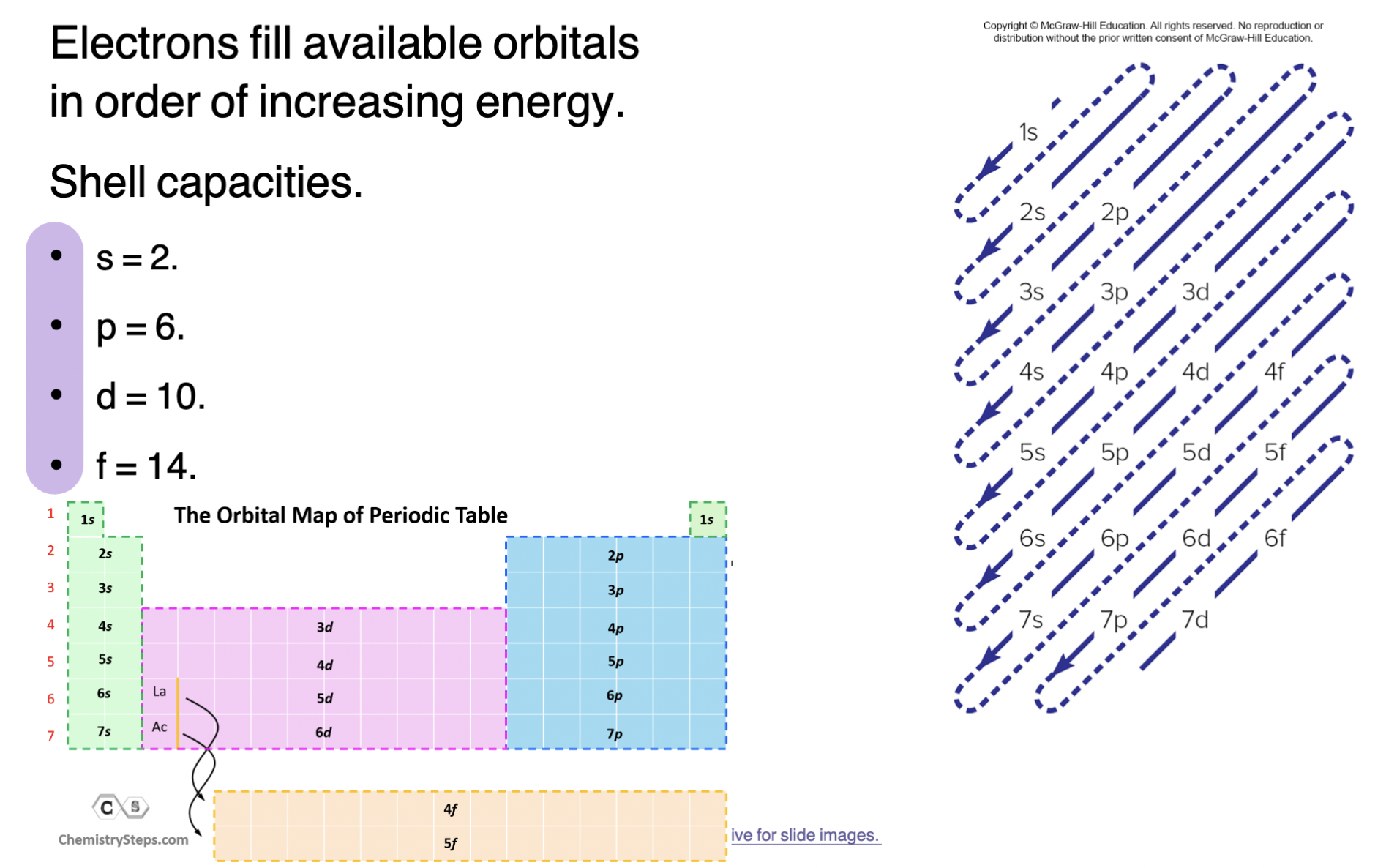
Electron Configuration
Arrangement of electrons into atomic orbitals
When writing them, electrons fill available orbitals in order of increasing energy
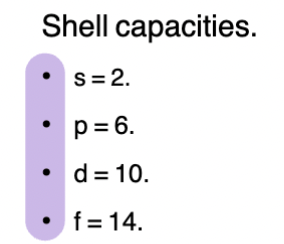
Picture
Periodic Table (Understand / Take info from it)
Periodic Chemical Properties
→ Rows = Periods
↓ Columns = Families or Groups
Electrons in outer orbits determine chemical properties
Valence electrons = Roman Numerals
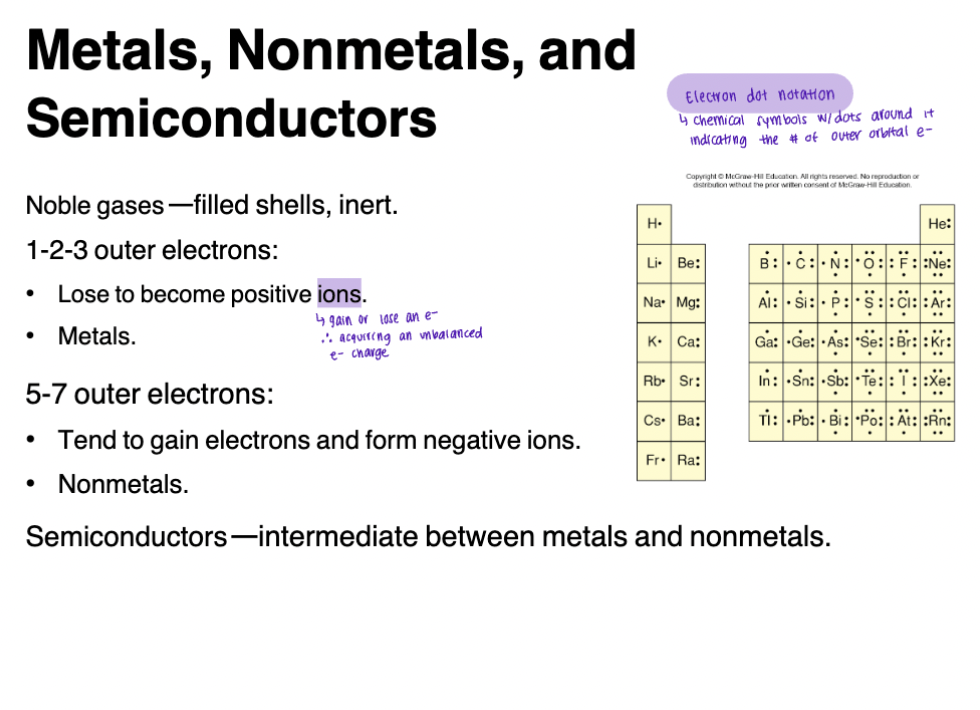
Alkali metals (IA)
Alkaline earths (IIA)
Halogens (VIIA)
Noble gases (VIIIA)
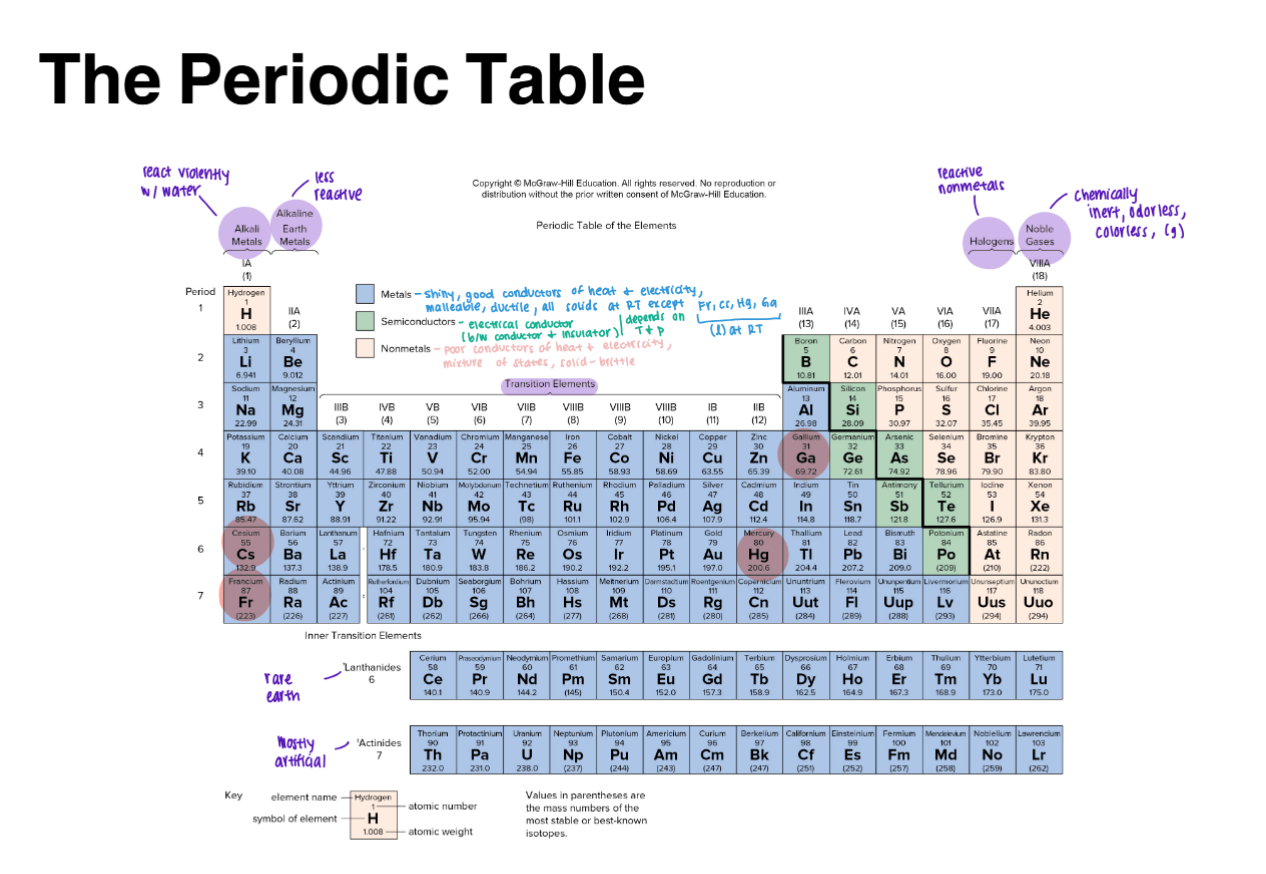
Metals, Nonmetals, Semiconductors