atomic_structure_review_
1/14
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Atomic Structure
Refers to the composition and arrangement of protons, neutrons, and electrons in an atom.
Nucleus
The very dense core of an atom that contains most of its mass, made up of protons and neutrons.
Electron Cloud
The region around the nucleus where the electrons are likely to be found; its mass is negligible compared to the nucleus.
Electrostatic Attraction
The force that holds the atom together, derived from the attraction between the negatively charged electron cloud and the positively charged nucleus.
Mass Number (A)
The sum of protons and neutrons in an atom's nucleus; represented as A = Z + N.
Atomic Number (Z)
The number of protons in the nucleus of an atom, which determines the element's identity.
Neutron Number (N)
The number of neutrons in an atom's nucleus.
Isotopes
Atoms of the same element that have the same number of protons but different numbers of neutrons, leading to different mass numbers.
Notation of Isotopes
Two common ways to write isotopes include the element's name followed by the mass number (e.g., Carbon-12) or the element's symbol with the mass number as a superscript (e.g., 12C).
Hydrogen Isotopes
Hydrogen-1 (mass number: 1), Hydrogen-2 (deuterium, mass number: 2), and Hydrogen-3 (tritium, mass number: 3).
Isotope Calculations
formula : •(Ar = %A x Ar(A) + %B x Ar(B) + %C x Ar (C)… etc) /100
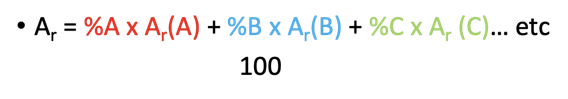
Relative Atomic Mass Calculation
A process to determine the average atomic mass of an element by factoring in the masses and relative abundances of its isotopes.
Abundance Calculation
The process of determining the percentage abundance of isotopes in a sample.
Copper Isotopes
Copper exists as Cu-63 and Cu-65, with relative abundances that can be calculated given the average atomic mass.
Lithium Isotopes
Lithium has two naturally occurring isotopes, 6Li and 7Li, with specific masses and their natural abundances can be calculated.