Chapter 7: Liquids and Solids
Comparison of Liquids and Solids to Gasses
- Liquids and solids are more dense than gasses
- Gasses expand to fill all available space and must be kept in a closed container. Liquids only fill container from bottom up and solids maintain their shape no matter what
- Gasses lack significant attractive forces.
Intermolecular Forces
Dipole-Dipole Attractive Forces
- Polar molecules are covalently bonded molecules that share electrons unequally. Sometimes called dipoles because of the one positive and one negative end.
- Dipoles orient themselves to have overall attractive forces between dipoles
- In the gaseous state, there is little attraction between molecules because they are so fat apart.
- For a gas to become a liquid, the attractive forces must overcome the kinetic energy of the moving gas molecule.
- Increasing pressure forces gas molecules closer together
- Decreasing temperature decreases kinetic energy
- Low boiling points indicate low attractive forces. High boiling points indicate high attractive forces.
- Highly polar molecules have high boiling points than molecules with less polarity
- Boiling point is the same thing as condensation point
London Forces of Attraction
- Molecules have constantly moving electrons which can cause momentary dipoles if the electrons are temporarily unevenly distributed. These are London forces and are very weak
- Sometimes called dispersion forces, instantaneous dipole forces, or induced dipole forces
- Argon, a noble gas, has all of it’s orbitals filled but the constant motion of electrons has a high likelihood of causing the electrons to be temporarily unevenly distributed, causing a dipole to form
- This is a very weak attractive force that allows argon to condense a very low boiling/condensation point
- Halogens also have no permanent dipoles but have higher boiling points than noble gasses. Not only that, but iodine is a solid and bromine is a liquid at room temperature
- This is because the halogens have a higher polarizability, or they have a higher likelihood of creating a dipole.
- Small atoms with tightly held electrons have low polarizability while large atoms with more loosely held electrons have a high polarizability.
- Compounds such as CH4, C2H6, C3H8, and C4H10 are called normal alkanes, n-alkanes. They all have the same general formula of CnH(2n+2).
- As the number of carbon atoms increase, so does the boiling point and increased chance of London forces
- Generally, more electrons means more opportunity for London forces which causes higher boiling points
Hydrogen Bonding
- n-alkanes are called homologous series because their formulas vary in regular fashion.
- Patterns can help chemists understand trends and physical properties
- Hydrogen bonds have extremely large dipole-dipole forces
- Only when hydrogen bonds to nitrogen, fluorine, or oxygen
- Chain-like structure forms when in the liquid state.
- HF has higher boiling point than NH3 because F has a larger EN (electronegativity)
- Water, with two hydrogens, can create two extra hydrogen bonds with neighboring oxygen because of the hydrogen and can bond with two more hydrogens because of the two lone pairs on the oxygen.
- This gives water a very structured network, giving it a higher boiling point than HF
Physical Properties of Liquids
Surface Tension
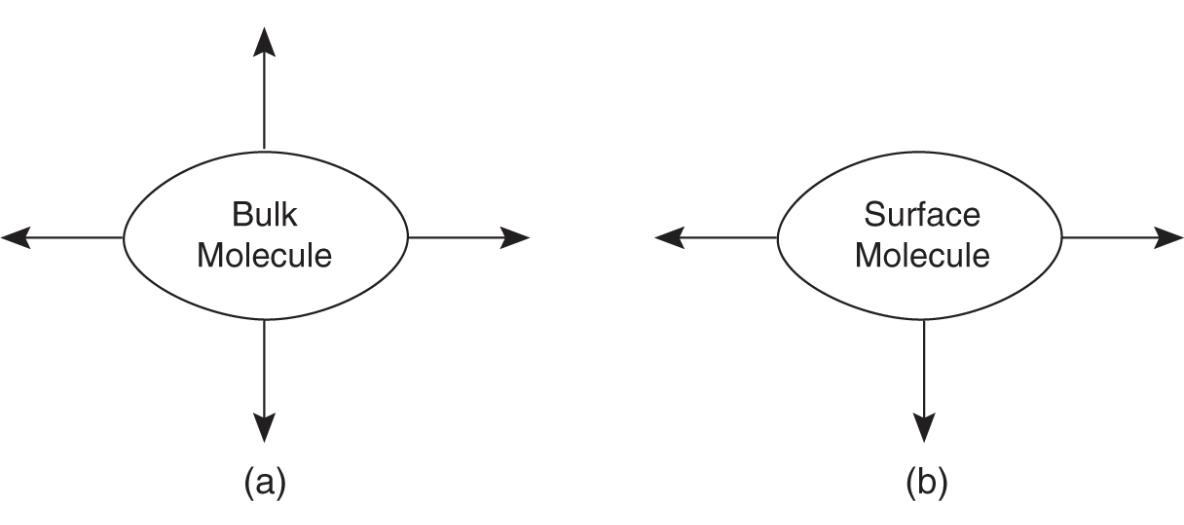
Surface tension is caused by an increase of attractive forces near the surface of liquid rather than near the center of the liquid
- This causes liquids to minimize their surface area, such as spheres forming when liquid gets on skin
- If an iron pin is placed gently on water, it will float, despite iron’s heavier density
The arrows indicate attractive forces
Cohesive forces are attractions between identical molecules in a liquid. Adhesive forces are attractions between different molecules, such as the liquid and a flat surface.
- If the cohesive is stronger than the adhesive forces, the liquid will bead. If the adhesive is stronger than the cohesive, the liquid will spread uniformly
Surfactants are chemicals that decrease cohesive forces, the surface tension. Surfactants are often found in dishwasher detergent to prevent water stains or beading.
Viscosity
- Viscosity is a liquid’s resistance to flow.
- Water has a low viscosity and flows easily. Syrup has a high viscosity and does not flow easily, especially when cold.
- Molecules with relatively low attractive forces have low viscosity.
- Viscosity usually decreases as the temperature of a liquid increases because they have a higher kinetic energy.
Evaporation
- Evaporation is when a liquid turns into a gas in an open container.
- Some liquids evaporate quicker than others. Gasoline evaporates quicker than water.
- Higher temperature can increase evaporation speed
- Evaporation is the reverse of condensation.
- Instead of attractive forces overcoming kinetic energy, there must be enough kinetic energy in order for the liquid to evaporate.
- Average kinetic energy is directly proportional to the kelvin temperature
- Kinetic energy is not even or the same between all molecules. Some are faster, some are slower than the average.
- Escape energy is the minimum kinetic energy needed for a molecule to become gas
- As long as the kinetic energy of a molecule is greater than the escape energy, it can become a gas.
- At a constant temperature, the proportion of molecules with enough kinetic energy to become a gas will remain constant and evaporate at a uniform rate until all molecules have entered the gaseous state.
- Surface area of a liquid is important when considering the evaporation rate because only the surface molecules can become a gas.
- If a liquid is in a narrow test tube, it will evaporate slower than if it was poured into a beaker or evaporation dish because of the surface area.
- Temperature plays an important factor. Higher temp means higher evaporation rate because of the higher average kinetic energy
Vapor Pressure
- Vapor pressure occurs when a liquid is a closed container and the pressure of gas formed above the liquid is measured.
- Liquids will become gas even in a closed container and only stops at a level that is dependent on the temperature. The final pressure is the vapor pressure
- As the liquid evaporates, the pressure increases and the liquid acts as a “wall”. When a gas particle hits the liquid, it will often condense rather than bounce. Initially, the evaporation rate is higher than the condensation rate. As more gas forms, the condensation rate increases and evaporation rate decreases. When these rates are equal, the vapor pressure has been reached. This is dynamic equilibrium.
Boiling Point
- Boiling occurs when the vapor pressure of a liquid is equal to the prevailing atmospheric pressure around that liquid.
- Boiling point is not constant unless the pressure is specified.
- Normal boiling point is used when the atmospheric pressure is 1 atm
- Decreasing pressure can decrease the temperature at which boiling occurs. This is vacuum distillation and is used to purify materials that would normally decompose at the regular boiling point or are generally heat sensitive.
Heat of Vaporization
- Heat of vaporization is the energy needed to convert 1 gram liquid to 1 gram gas at a temperature equal to the normal boiling point of the liquid.
Solids
Crystal Types based on Attractive Forces
Metallic Crystals
- Metallic crystal is visualized as a rigid structure of metal nuclei and inner electrons. Valence electrons are very mobile moving freely from atom to atom and help bond metal atoms together with different degrees of force.
- Melting points can vary from 1000 degrees celsius to below room temperature and help measure attractive forces in the metal because melting disrupts crystal bonding.
- Lattice energy is the energy need to disrupt a crystal
- Mobile valence electrons explains the ability of electricity and heat conduction.
- Electrons quickly carry charge and thermal energy throughout the metal.
- Electrons interacting with light can affect the color
- Atoms can be moved from one position to another without disrupting the crystal in a major way. Because of the available movement, metals are malleable and have varying softness, hardness, and brittleness. Alloys can be formed to change these properties
- Interstitial alloy occurs when spaces between atoms are filled by an atom much smaller than it.
- Volume is hardly changed when an atom takes up space but adds mass, so the density is increased.
- Increases total attractive forces because there are new attractions
- New alloy is stronger and harder than the original materials
- Stainless steel (iron and carbon)
- Substitutional alloys occur when an atom is replaced with another atom of similar size.
- The new and replaced atoms have similar attractive forces.
- Substitutional alloys have properties that are an in-between of the two combined metals
- Silver-gold alloy will tend to be soft, ductile and has a density in between silver and gold
Ionic Crystals
- Almost all ionic compounds are solid with rigid crystalline structures (lattices). A large lattice energy is required to separate the ions. This high lattice energy causes ionic compounds to have high melting and boiling points.
- Ionic crystals have a lattice structure of alternating positive and negative ions. Size of the crystal is affected by ion sizes and how close the ions are
- Ionic crystals are rigid and brittle because of the alternating charged ends.
- In a metallic crystal, if the crystal is hit, the atoms will shift but the bond is not disrupted
- In an ionic crystal, the shift in atoms causes the charges to become repulsive rather than attractive.
Molecular Crystals
- Molecular crystals are composed of nonmetal atom or covalent molecules.
- Held together by London forces, dipole-dipole attractions, hydrogen bonds, or a mix of these.
- Weak bonds cause molecular crystals tend to be soft, with a low melting point.
Network (Covalent) Crystals
- Network crystals have a lattice structure of covalently bonded atoms.
- Network covalent crystals are very hard.
- Diamonds and quartz are both network covalent crystals
Amorphous (Noncrystalline) Substances
- Some materials are amorphous and don’t form crystals
- Don’t have a distinct melting point but gradually get softer over large temperature range
- Ordinary glass
- Many plastics, or polymers, are part crystalline and part amorphous
Phase Changes
Heating and Cooling Curves
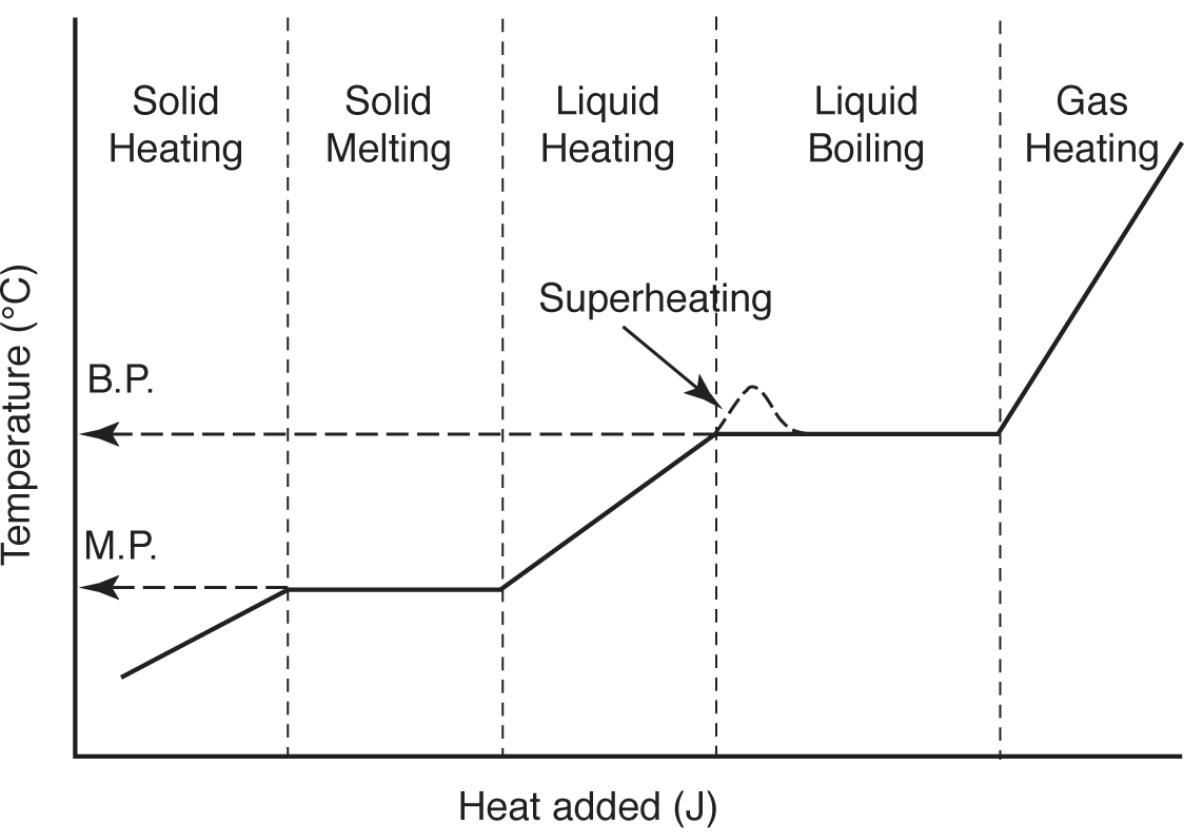
Adding heat to a solid material at a constant rate causes the following effects:
- Temperature of a solid will increase at a constant rate until the solid starts to melt
- Temperature will stops rising until the solid is completely a liquid
- Temperature of liquid increases at a constant rate until boiling starts
- Temperature remains constant until all liquid is a gas
- Temperature of gas increases at constant rate
This process is called the heating curve
The heat capacity is the reciprocal of the slope where the temperature is increasing
Specific heat is the heat capacity divided by the grams of the sample used
The molar heat of fusion (melting) is the length of the solid melting plateau divided by the number of moles of the sample.
- Enthalpy of fusion
The molar heat of vaporization is the length of the liquid boiling plateau divided by moles
- Enthalpy of vaporization
Cooling curve is the reverse of the heating curve.
- Condensation point and crystallization point is replaced with boiling and melting point
Some liquids can be supercooled where a liquid is cooled below melting point and remains a liquid.
- Supercooled liquids are in a metastable state where it will crystallize rapidly if shaken or by adding a seed crystal.