Neuroscience; week 9; clinical trials and drug discovery
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
What is a clinical trial? and what can they be used for?
Clinical trials are medical research studies involving people
They can be used for different reasons:
Prevent disease and reduce the number of people who become ill (disease management)
Treat illness and improve or increase the number of people cured
Improve the quality of life for people living with illness –
reducing symptoms or side effects, rather than the disease
For disease diagnosis and health problems
help to diagnose the disease earlier
Not always about new medicines but could ‘interventions’ to modify lifestyle or behaviour
such as cognitive therapy
A brief history of clinical trials:
Evidence of clinical trials been known for centuries
Recorded in the ‘’Book of Daniel’’ in The Bible
King Nebuchadnezzar ordered his people to eat only meat and drink only wine – to keep them strong
Several young men of royal blood objected as they only ate vegetables, so the king allowed them to eat legumes (beans, peas, chickpeas, lentils) and water for only 10 days, compared to a group that ate meat
After this point, the vegetarians appeared better nourished than the meat eaters
Although not a clinical trial first example of human experiment guiding a decision about public health using different groups of people
1747 Scurvy trial by James Lind
First clinical trial in the modern era
Scurvy was a terrible disease that effected sailors on ships.
Lind thought it was related to diet.
He had 12 sailors with scurvy and treated them with separate food supplements, the two sailors who had oranges and lemons recovered very quickly and a third who had cider was the next best.
Wrote ‘’Treatis on Scurvy’’ published in 1753 : Not only first description of a controlled trial but also a systematic review of previous literature on scurvy, so made sure he didn’t just repeat what was already said.
It took the British Navy 50 years to adopt these findings in full!
Edward Jenner 1700’s side stepped the process:
Jenner was a rural GP in England – for years he had been taking notes and making observations from his local patient population.
He noticed that all the milk maids on the farms did not contract the deadly small pox disease and all milk maids reported that they had all previously caught a similar disease called cow pox – which was a lot milder with no lasting effects, which gave them immunity
In May of 1796 – Jenner performed the first ever experimental vaccination on an 8 year old boy called James Phipps. He had escaped small pox so far, Jenna infected him with cow pox then exposed him to small pox. The cow pox exposure saved him from the small pox.
The experiment worked but If this was done today, the scientist would face jail, However, it is argued this experiment led to Modern vaccination
When Jenner presented his findings he was rejected and told to collect a larger sample data set, he even include his own son.
Finally published : Inquiry into the Causes and Effects of Variolae Vaccinae (1798)
But the finding was still wildly ridiculed in society especially among religious groups. This was a time of science Vs religion.
the impact of Jenner’s vaccination method
It was only in the 1800’s that major hospitals across Europe started to do studies (‘clinical trials’) and that Jenner’s vaccination method could be robustly assessed
This led to the development of modern vaccination and laid down the foundations for the rigorous clinical trials we see now.
Jenner’s legacy was huge (think of polio, AID’s, MMR, even COVID19)
Plecebo
first definition, Hoopers Medical Dictionary of 1811:
‘’An epithet given to any medicine more to please than the benefit of the patient’’
1863 : USA Medic Austin Flint first used a placebo in a clinical study
He gave a ‘pleceboic remedy’ for rheumatism to patients – all reported positive effects
Even though it did nothing to treat the disease.
1943- trial
1943 : The First double blind controlled trial – Patulin (related to penecillin for common cold)
Carried out by the Medical Research Council
Recruited over a 1000 subjects from British offices and factory workers suffering from the common cold – difficult in wartime
Both the Dr’s and patients were blinded to the treatment
Unfortunately the results failed to show and effect
1946- trial
1946 : First Randomised curative trial – using Streptomycin to treat tb
carried out by the Medical research council
Patients had systematic and randomised enrolment
rather than alternating into treatment and control groups.
Dr’s looking at the x-ray results were blinded to the different patient groups.
This study set the ground work for the basis of clinical trials we see today.
included the establishment of national and international regulatory frameworks.
Why are clinical trials important?
Health professional need evidence to compare approaches, clinical trials are good way of comparison
Without clinical trials:
Patients cold be given medicines that do not work
Wasting resources, giving ineffective drugs to patients
Medicine could even make the patient worse
Who is in control of the clinical trial process?
Generally designed by doctors and other specialists (generally statisticians) but involve a wide variety of people:
Doctors, nurses, patients, statisticians, trial managers and representatives from pharmaceutical companies to design the best possible trial
Designed to offer the least risk to the patient but maximum potential new treatment or intervention being tested.
Generally start with a systematic review of previous trials performed in the same area of disease of using similar drugs – To assess whether this research has already been done.
Based on all of this information all the involved parties get together and design the trials protocol
Gaining ethical approval
The trial protocol is sent to a research ethics committee
Independent group of people that includes doctors, nurses, other medical staff, members of the public and sometimes lawyers
They decide whether the trial is ethical:
They will focus on:
Do the potential benefits of the treatment/intervention out way the costs
The information provided to the participants in the trial is clear and satisfactory
That will people will be approached in an appropriate way
Is compensation in place for patients if something goes wrong
Travel expenses – are they in place
The trial can only start once ethical approval is in place
Clinical trials need a sponsor
Sponsor: Individual, company, institution or group of organisations that are responsible for the initiation, management and financing of the research.
All research under the remit of Secretary of State for Heath must have a formal sponsor.
Sponsorship of involving medicines
It is a legal requirement for any clinical trial of an investigational medicinal product (CTIMP) to be sponsored. This includes provision for insurance in case things go wrong.
Clinical trials can be split into 4 distinct phases
Phase 1 : Early Stage – Generally small groups of healthy subjects but sometimes patients – Used to test how safe the treatment is are there any large side effects
Phase 2 : By this stage allot more is known about the treatment
This will now be tested in a larger group of people to asses safety and side effects in greater detail
For the first time to see if the treatment has a positive effect in patients
Phase 3 : Moves up to hundreds if not 1000s of people often international groups of people
Compare the new drug to a standard treatment
How well drug works and how long the effects last for
Finds out more about any serious side effects and how long they last for.
Phase 4 : The drug is now licensed and being used as a treatment
Gets stats on how well the drug is working on a large population
Any long term risks and benefits
Rare side effects.
Controlled trials
Designed to compare different treatments
Usually two groups – trials group – given new treatment
Control group given standard treatment –
Where no standard treatment - the control group may not be given any treatment or may be given a placebo
What happens during a trial?
As well as test to assess whether the treatment is working the researchers will also assess
Any potential side effects
Any new symptoms
Wider effects of treatment such as quality of life, day to day activities
Your mental state is the treatment making you happy, sad, anxious or depressed?
Cost effectiveness of treatment – are you able to work, how often you need to visit the doctor
What happens at the end of a trial?
Normally trials can last for many years (although as can be seen with COVID19 the process can be dramatically speeded up)
All participants will have access to the results of the trial if they want them.
They will also be published to help other researchers in the field and allow advancements to be taken up by everyone
In some instances the treatment used as part of the trial may not be available on the NHS – at the end of the trial you will be given the standard treatment.
In some cases you may be able to buy the new treatment
All you information will be kept confidential : a key requirement of the trials process.
What happens if something goes wrong in the clinical trial?
Before the start of any trial arrangements need to be put in place in case something goes wrong an people are harmed
Ethics committees can refuse permission if this is not in place
Important for participants to know that insurance is in place before the trial starts.
Thalidomide: 1960s
One of the darkest episodes in pharmaceutical research history
The drug was marketed as a mild sleeping pill safe even for pregnant women in the late 1950’s
As it seemed to reduce morning sickness, may pregnant women took it
However, it caused thousands of babies worldwide to be born with malformed limbs.
During the testing process on animals – no tests were included to look at the effects on pregnancy.
The damage was revealed in 1962 – before then every new drug was seen as beneficial
However, Thalidamide has been shown to reduce the symptoms of leprosy and is still being used to treat the condition.
In the USA : the boss of the Federal Drug Administration - Frances Kelsey – despite huge pressure from the pharmaceutical industry refused a license – John F Kennedy praised her as a national heroine.
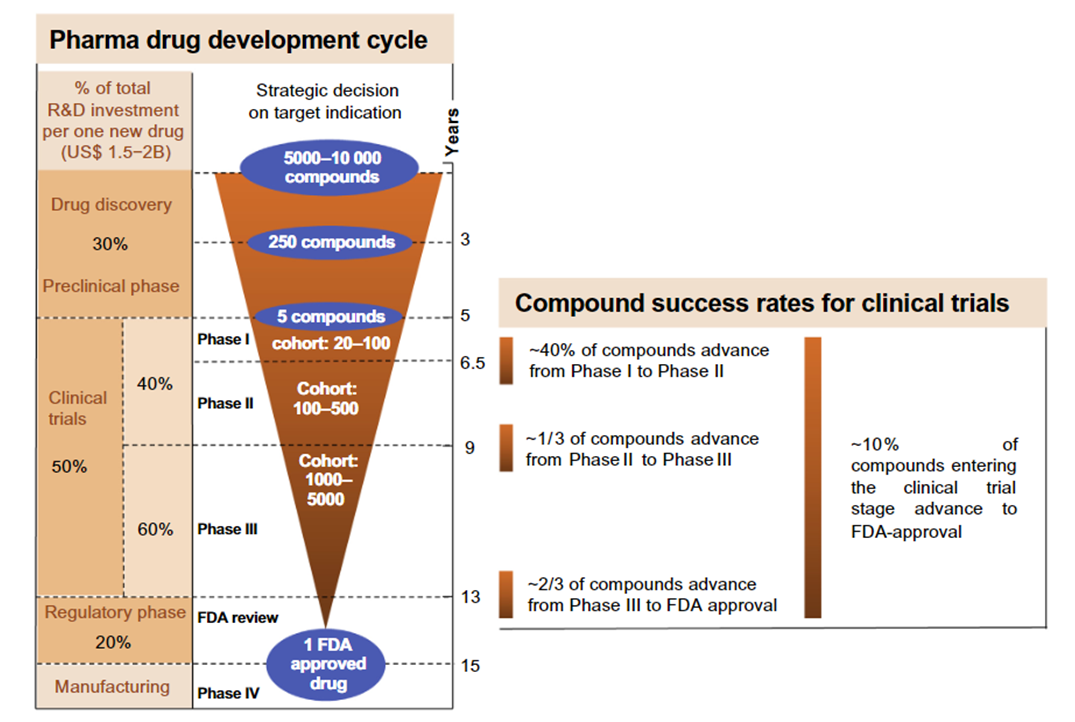
Cost and success rate of clinical trials
Clinical trials are expensive
It was calculated that the average cost of a 5.5 year (non-pharmacological) clinical trial involving collecting data across 20 centres in the UK (stage 2 or 3) would cost on average ~£1M to administer:
Staff needed were the highest costs, including:
Managers
Researchers
Statisticians
~30% costs for non-staffing expenses such as ethical approval
In the UK the actual price of taking a drug from development to the market is £1.1 Billion
Only one in 10 drugs make it through to stage 4 in clinical trials …
Partially explains why drugs that developed are expensive because the companies have so many failures.
Drug discovery
Inextricably linked to clinical trials is another process called
Drug Discovery - this aspect is just as expensive as the clinical trials that follow them and also has a high failure rate.
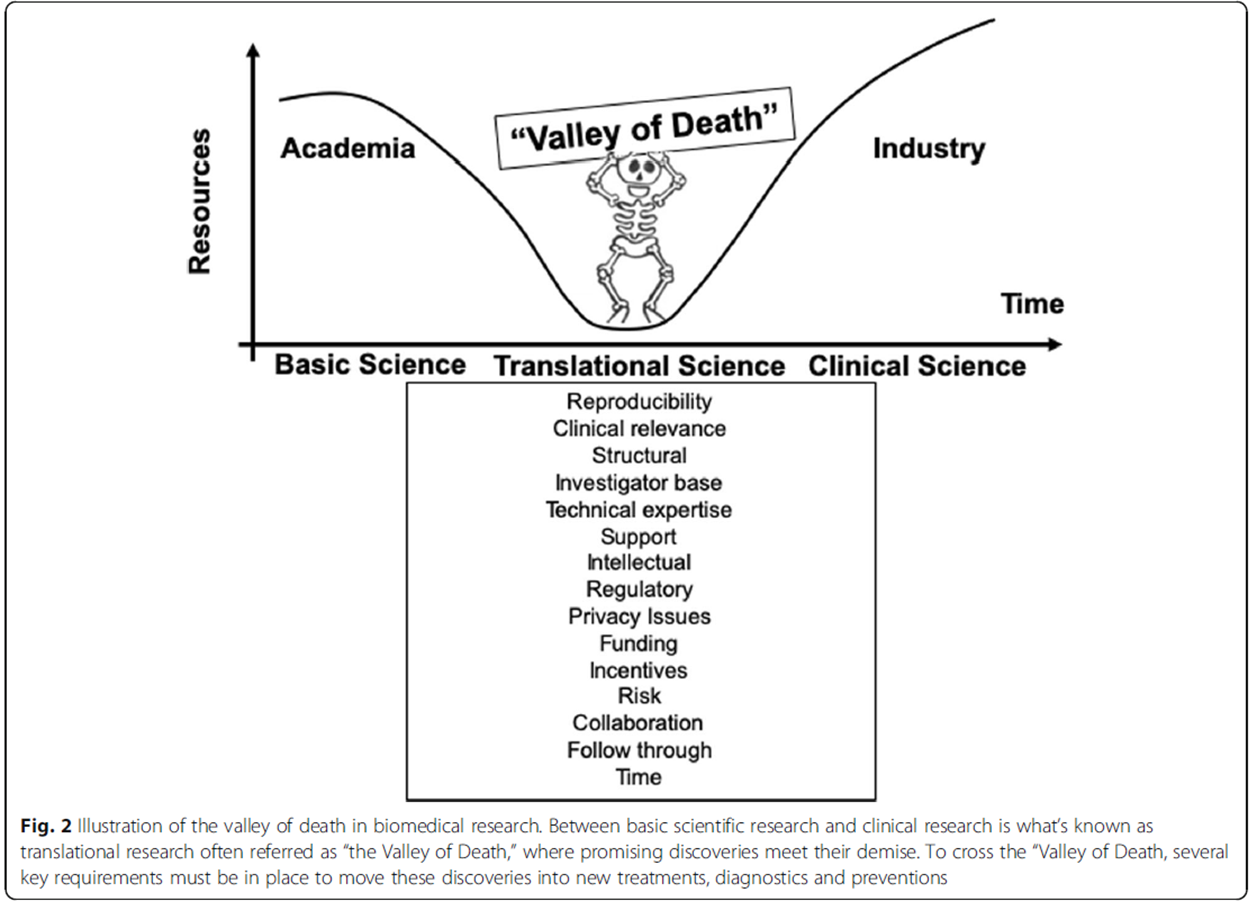
This process of going from drug discovery to developing a new medicine has been termed the valley of death
The valley of death
The gap between the development of the drug from basic science research into the clinical trial pipeline. This is the valley of death, every 10 drugs that get through from T0, only one gets through to T4
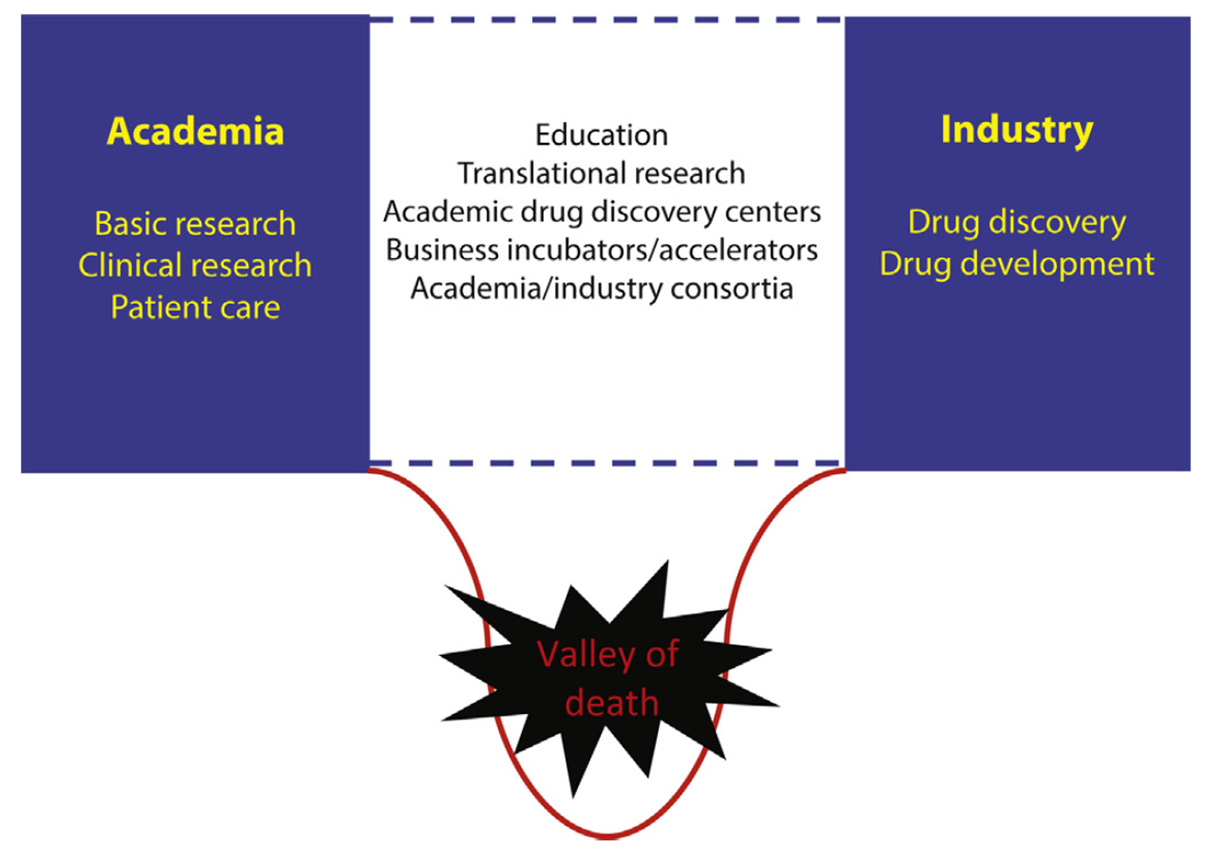
Astroseneca create a drug library to release for free to test on researchers experiments- in hopes to reduce the valley of death
Typically researchers discover new drugs through
New insights into a disease process allow researchers to design a product to stop/reverse the effects of a disease
Many screenings of compounds to find the possible beneficial effects against any of a large number of disease
At this stage thousands of compounds may be candidates for development.
After early testing only a few compounds will look promising and call for further study
Existing treatments that have an unexpected effect against a new disease (re-purposing or orphan drugs - could be implemented quickly at clinics as they were already safely tested) – this is true for treatments of COVID19 as vaccine methods were already proved safe.
New technologies that provide new ways to target the medicine to specifics sites within the body – ie across the blood brain barrier
Example of a method of drug screening
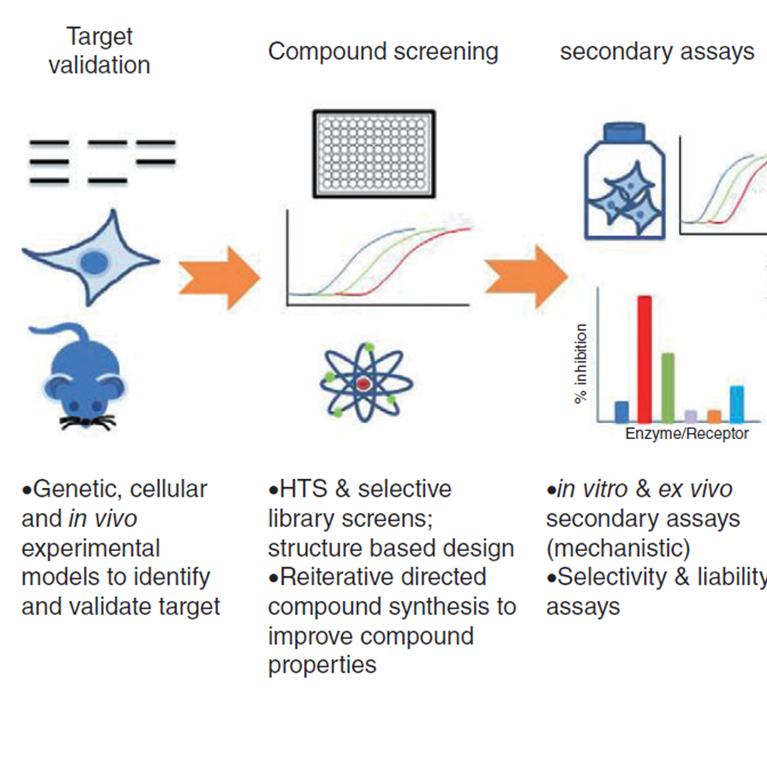
Target validation:
what cell, organism or disease are we trying to work on
Compound screening:
basic testing to see whether the compound you’ve made or tested actually have an effect on your target
High throughput screening (HTS),
1994: 96 wells
1998: 384 wells
2000: 1536 wells
all to test thousands of compounds at a faster simultaneous time
Secondary assays:
may group cells together to test effects or on brain tissue
Once researchers identify a promising compound for development, they conduct experiments to gather information on:
How it is absorbed, distributed, metabolised and excreted
Its potential benefits and mechanisms of action
The best dosage
in animal studies, you can keep increasing the dosage until half the animals die to identify the best dosage
The best way to give the drug (such as by mouth or injection)
How toxic is the drug
How it interacts with other drug and treatments
one of the biggest problems is that most people with a disease like dementia have another issue first like heart disease, we need to see how drugs for these diseases interact with each other and change their efficacies and symptoms (they acc found that giving their lab mice dementia after heart disease eased their heart problems??)
How it compares to existing drugs
Development: In Vitro and in vivo testing
Before testing the drug on people, researchers must see if it has the potential to cause serious harm (toxicity)
Two types of pre-clinical research are
In vitro – looking at how cells in a test tube are effected by the treatment
In vivo- involving small animals like mice, but for some studies especially brain diseases, non-human primates may be used at later stages
There are strict guidelines for pre-clinical laboratories to adhere to- referred to as Good Laboratory Practices (GLP)
GLP aims to standardise approaches and method
Good and important laboratory practice
GLP sets minimum basic requirements for
Study conduct
Personnel – training of staff
Facilities
Equipment – safe and rigorously checked
Written protocols for all experiments
Standard operating procedures
minimising experimenter error, all members across different labs are given the same standards
Clearly writing study reports
Quality assurance oversite for each program of work - essentially ethical approval
Usually, pre-clinical studies are not very large. However, they must provide detailed information on dosing and toxicity levels.
After pre-clinical testing, researchers review their findings and decide whether to proceed to clinical trials.
Why do clinical studies fail? Example from stroke research
Over the past 40 years, billions have been spent on stroke research, but only one drug has come to market and it is only when a stroke is occurring, that you then inject them. Some of the other drugs work but only if you knew if the stroke was going to happen, but this is a spontaneous event in humans.
Accurate and repeatable strokes can be caused in rodents.
However as systematic review of the literature showed that
Over 800 drugs have been tested on animal models
500 of these work in reducing the effects of the stroke
100 went to clinical trials
Only one drug has become a treatment!
Researchers who randomised and blinding the experiments had less favourable results
Of 100 studies looked at in a separate study only 36% were randomised and 11% were blinded ---- these are routine in clinical trials
Why do clinical studies fail? Example from Alzheimer’s
Despite billions of pounds in investment – no disease modifying Alzheimer's drug has been developed
Could be the wrong target – most studies focus on Beta Amyloid plaques – it could be something else – e.g. the blood supply
Interventions might be to late - the damage is already done
Early biomarkers needed – existing treatments could be re-examined
Clinical trials often less than 5 years – for Alzheimer's this might not be long enough – however a longer trial is far more expensive
Not easy problems to solve.
Large co-hort human studies may help especially in early biomarker detection
Co-hort studies- alzheimers
There are many large co-hort studies- one of the largest is ALZHEIMERS DISEASE NEUROIMAGING INITIATIVE (USA)
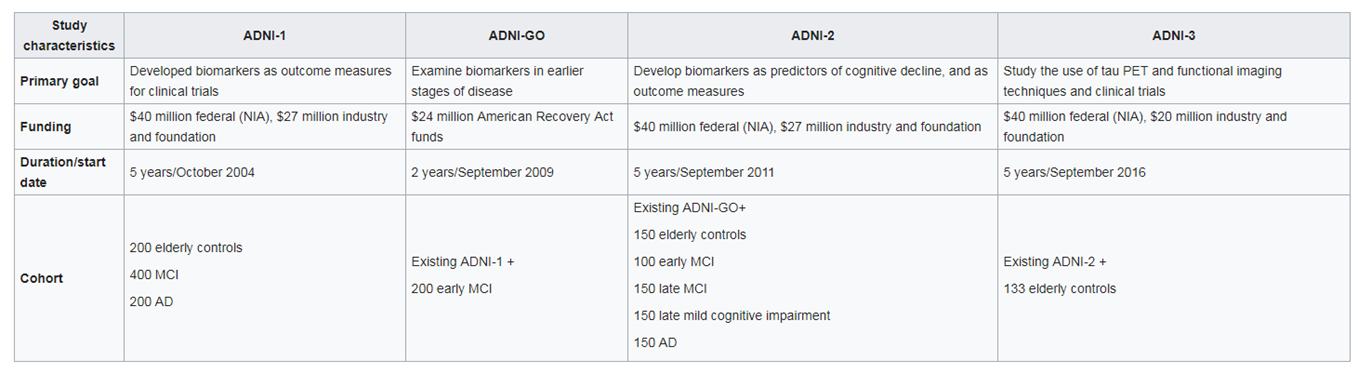
Budget so far $218M : multi-modal data from elderly controls and AD patients. Tests include:
Detailed history of patients and assessment of health and education
Neuropsychological tests
Genetic testing for risk factors :APOE4
Lumbar puncture :CSF measurement
MRI both structural and function scans
PET for glucose consumption, Tau and Beta Amyloid
Post mortem histology
BUT is this approach working?
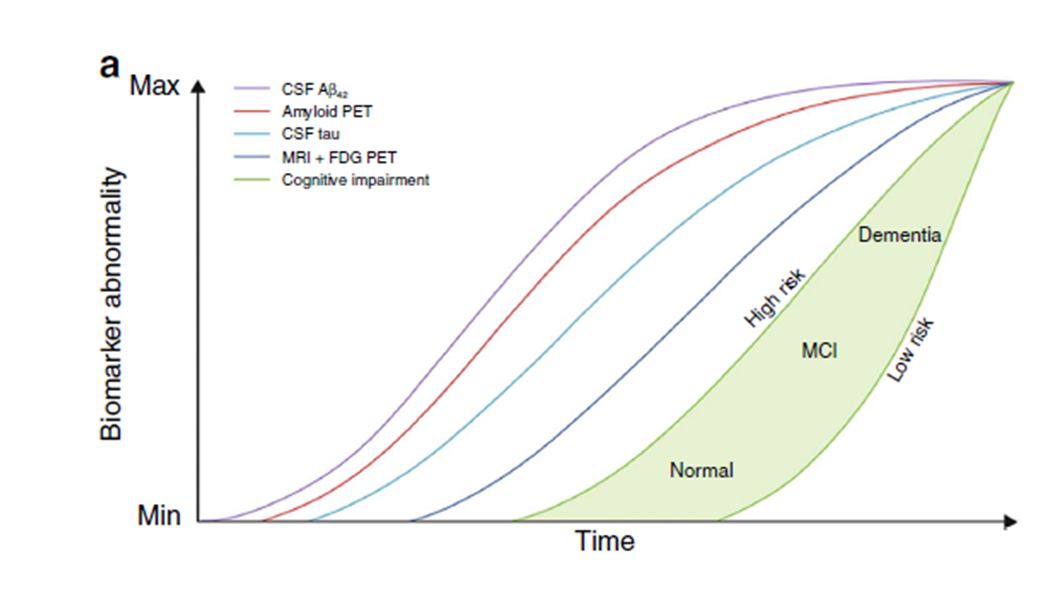
This theoretical figure is what should be produced by all the ADNI studies. It is a complete time line of disease progression (it is hypothetical) and has potential to detect early biomarkers and critical time points for intervention
Currently 3400 papers have been published from ADNI studies and it keeps growing
These big cohort studies cost a fortune, if needed they will take out funding from smaller labs to fund them. The cerbrovascular system goes first!
Now moving from theoretical to measured responses- LOAD


LOAD- 80% of people with dementia is sporadic not genetic, life style is important, like a balanced diet can reduce chance by 35%
Mathematical analysis of biomarkers:
Anybody to get LOAD, the first emergent biomarker is the blood vessels in the brain. This created a new version of research for vascular based therapy.
Clinical trials in the future
All about increasing the efficiency of all the steps in the process will save millions and importantly will save time,
There is a massive amount of failure down the chain, to make it more efficient we can increase the number of drugs and reduce the time, saving a fortune, this could be helped by AI
Using more targeted approaches to select more targeted patient populations: genome studies
AI could
optimise cohort composition,
maximise chances of successful outcomes),
to see which patients will stick through to lower dropout rates,
better planning of the trial to make it more effective
making things faster to make it cheaper
to decipher which patients will follow the rules better (adherence)
Challenges:
EMR data harmonisation
data privacy, integrity and security
explainability of AI
The case of COVID 19- how can the process be speeded up? What is the process?
Standard protocol of developing a vaccine takes 10 years and $500 million
Answer: Finance and parallel infrastructure development
Doing steps simultaneously dramatically reduces time
A fantastic success story for science and should make us better prepared for pandemics in the future
This new tech is being rolled out for many diseases-
malaria, HIV and cancer
There are 14 vaccines approved and in use, 30 vaccines in stage 3 clinical trials for COVID
Discovery research:
up to 100 potential vaccines
2-5 years
Pre-clinical
20 potential vaccines
2 years
clinical development
phase I: is it safe?
10 potential vaccines, 1-2 years
Phase II: Doe sit activate an immune response?
2-3 years, 5 potential vaccines
Phase III: Does it protect against the disease?
2-4 years, 1 potential vaccine
regulatory review and approval
1-2 years and 1 vaccine
what contributes to the valley of death?
reproducibility
clinical relevance
structural
investigator base
technical expertise
support
intellectual
regulatory
privacy issues
funding
it is difficult to get grants
incentives
risk
a lot of researchers in france are being paid to do safe slow science to get two papers published per year
collaboration
collaboration between pre-clinical and clinical researchers is difficult
follow through
time
T0
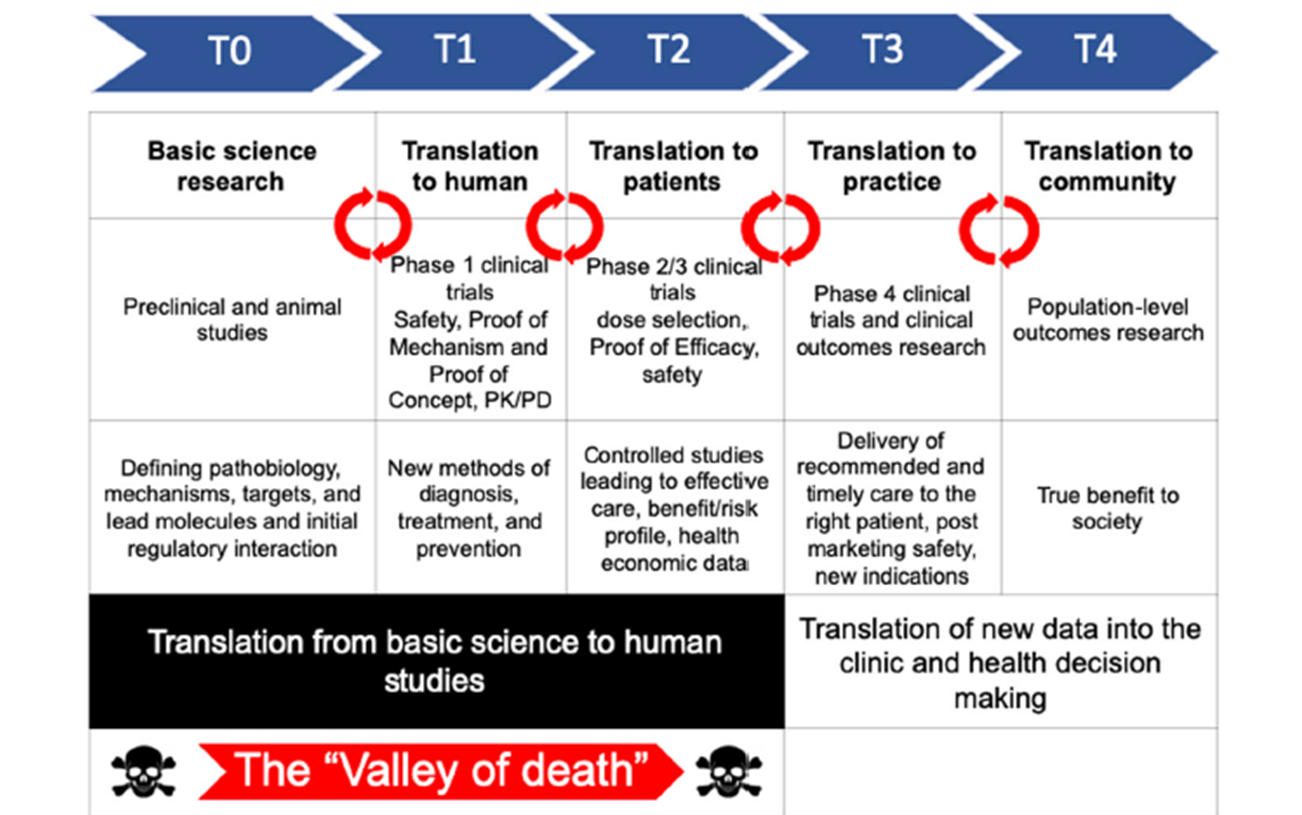
Basic science research
preclinical and animal studies:
Defining pathobiology, mechanisms, targets and lead molecules
AI in clinical trials
Clinical trials cost a fortune so AI can be used to think of grant proposals, researchers were found if they don’t use AI for grant proposals in the next five years, they won’t get any funding because every grant will be polished by them.
T1

Translate to humans:
phase 1 clinical trials
safety, proof of mechanism and proof of concept
new methods of diagnosis, treatment and pervention
T2

Translation to patients
Phase 2/3 clinical trials
dose selection
proof of efficacy
safety
controlled studies leading to effective care, benefit/risk profile, health economic data
T3

Translation to practice
Phase 4:
clinical trials and clinical outcomes research
Delivery of recommended and timely care to the right patient, post marketing safely, new indications
translation of new data into the clinic and health decision making
T4

translation to community
population-level outcomes research
true benefit to society
translation of new data into the clinic and health decision making
Pharma drug development cycle