MLT 118 Exam 3
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
10^-3 or ( 0.0001)
mili-
10^-6 or (0.0000001)
micro-
10^3 or (1,000)
Kilo-
450 mg ____(g)rams
0.45 g
250ųL _____ mL
0.25mL
mmol/L formula
mmol/L = (mg/dL)/(GWM) x 10
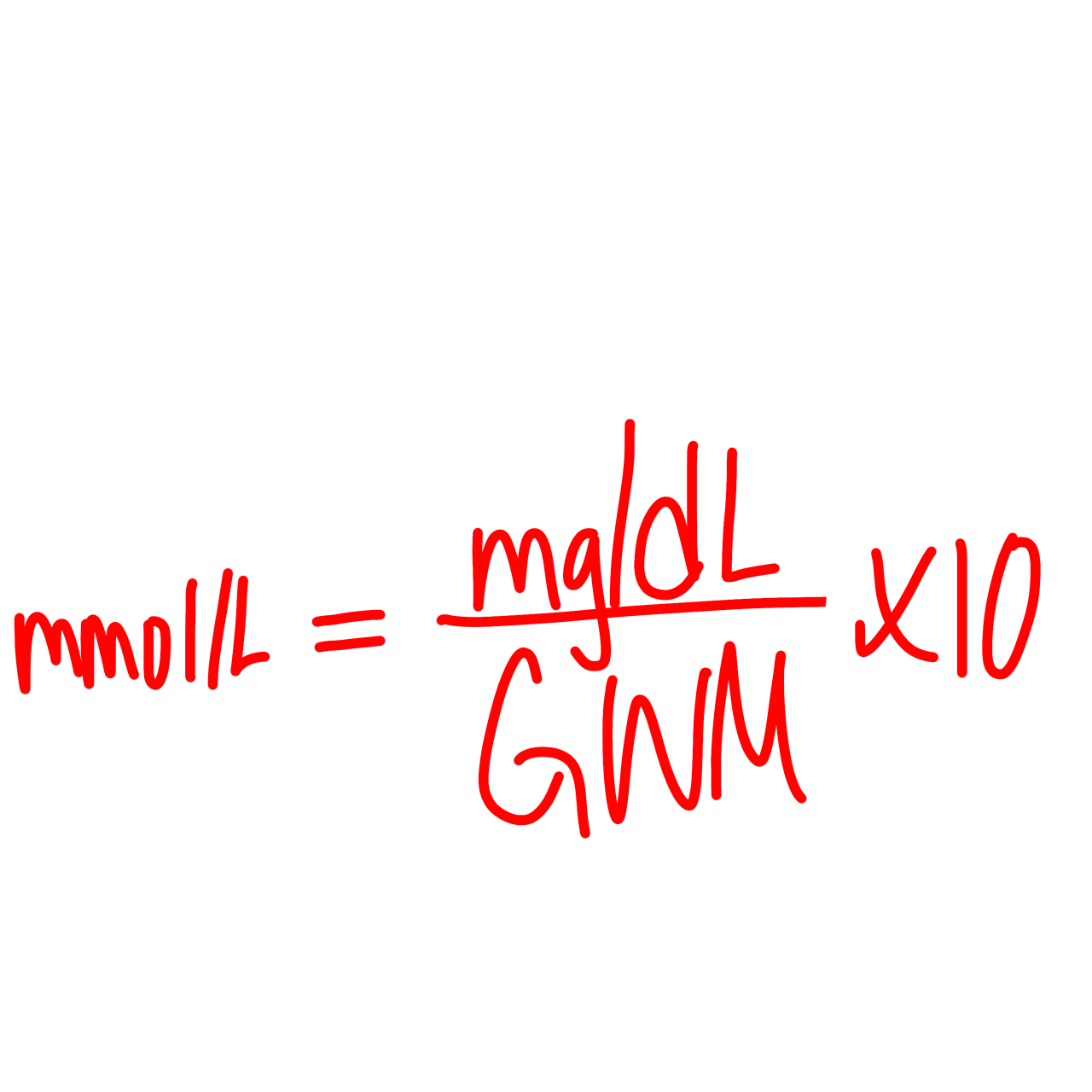
MEq formula
mEq = (mg/dL)/(GEW) x 10
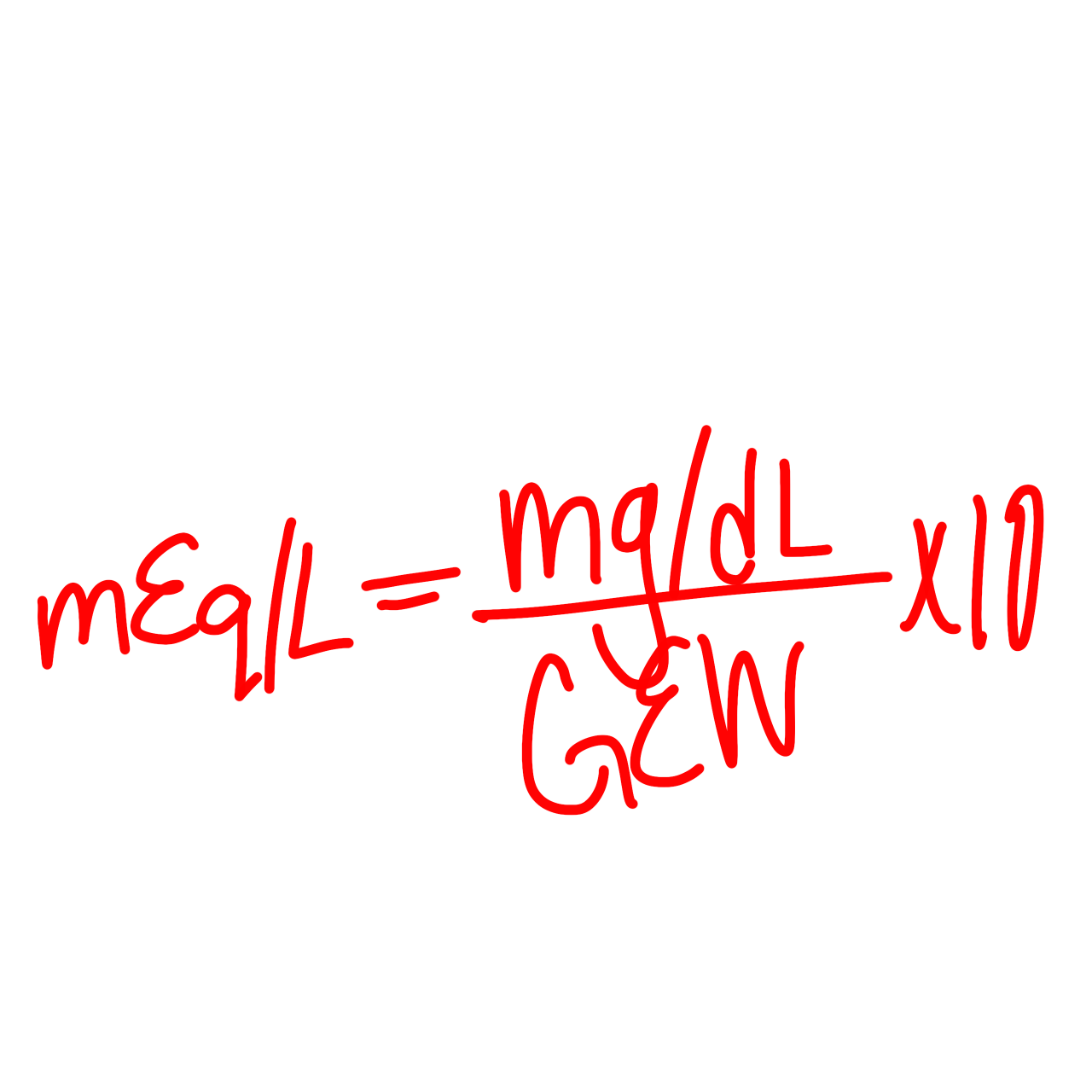
℃ to ℉ formula
T(F) = (1.8 x T(C)) + 32
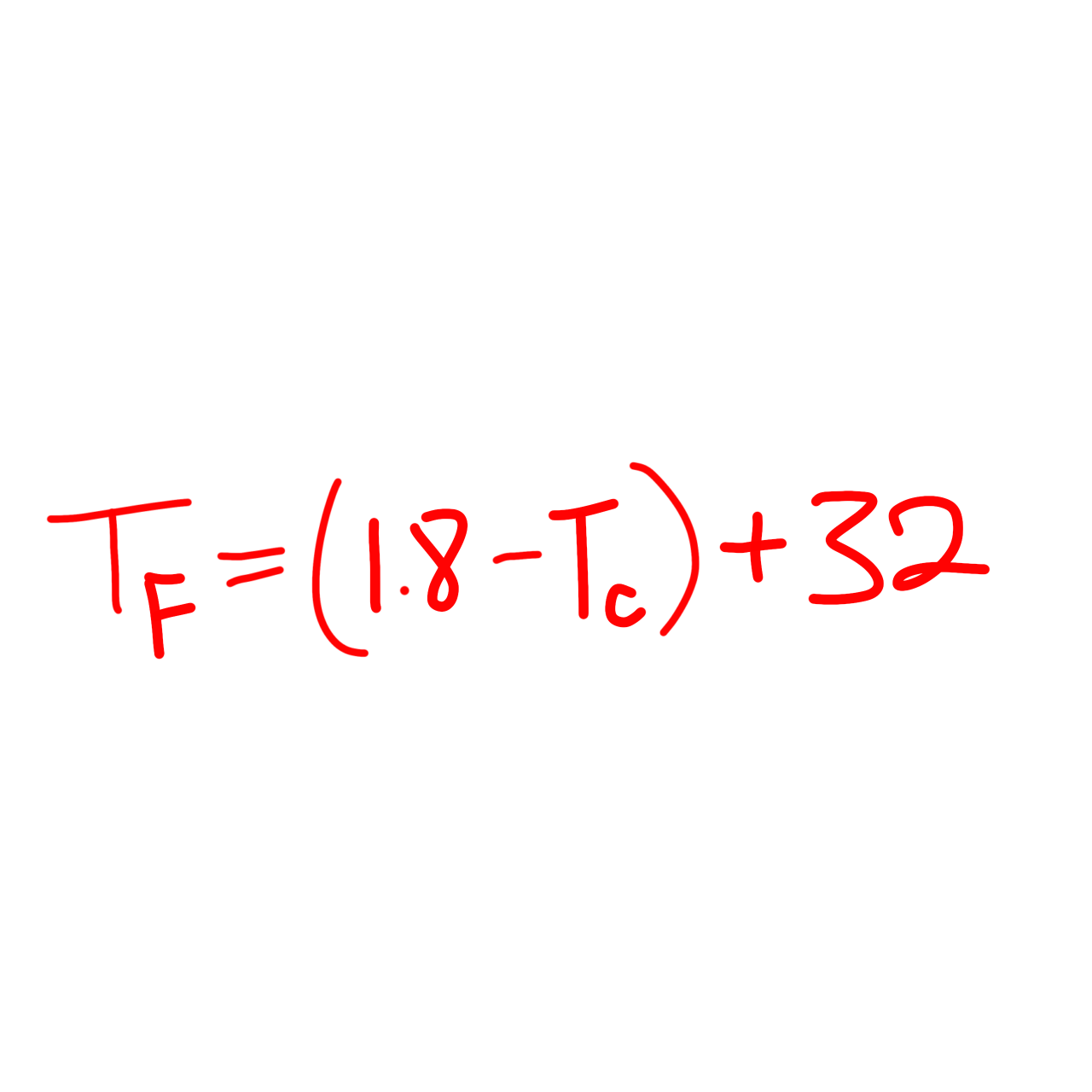
Solve. 20℃ to ℉
68℉
℉ to ℃
T(C) = (T(F) - 32)/1.8
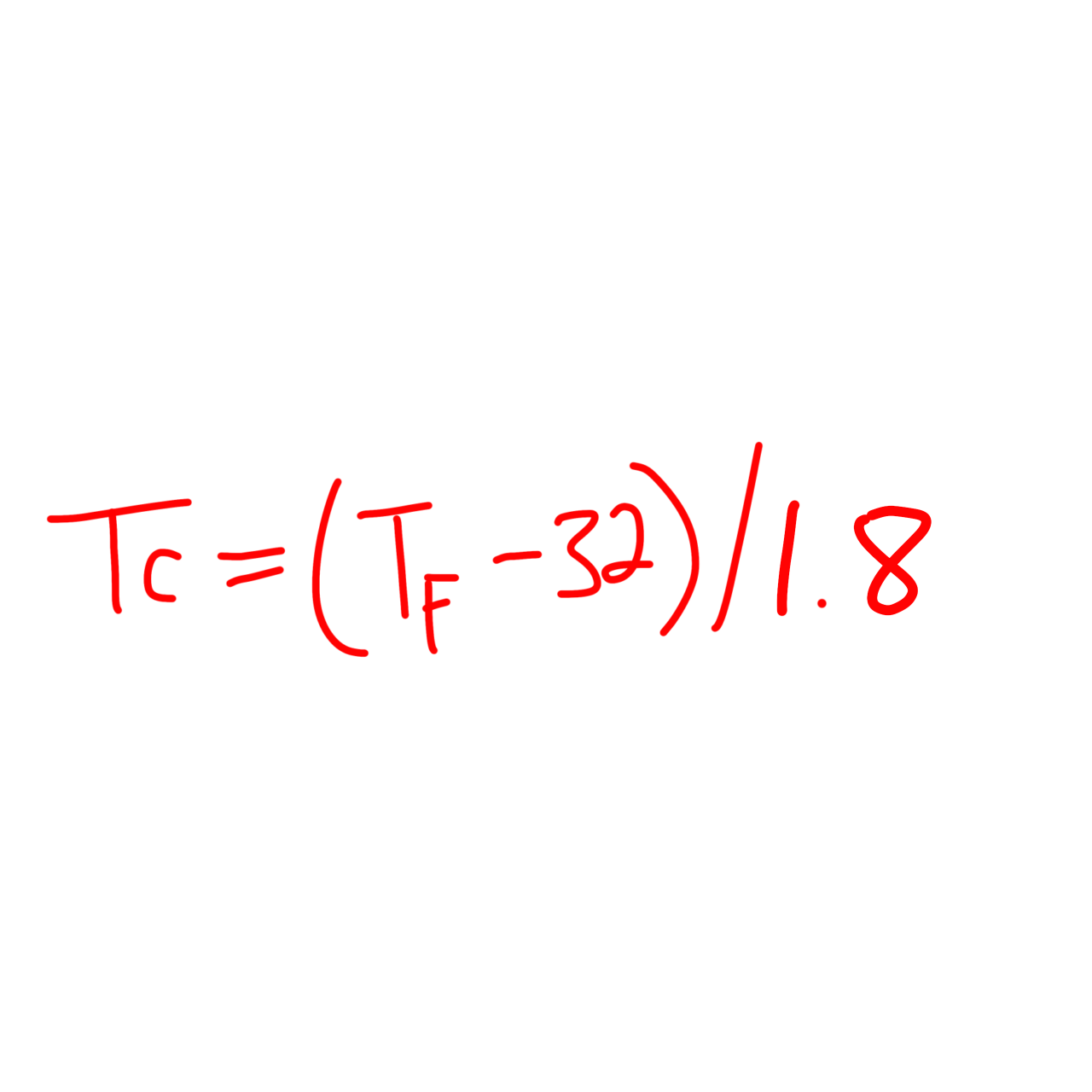
Solve. 90℉ to ℃
32.2 ℃
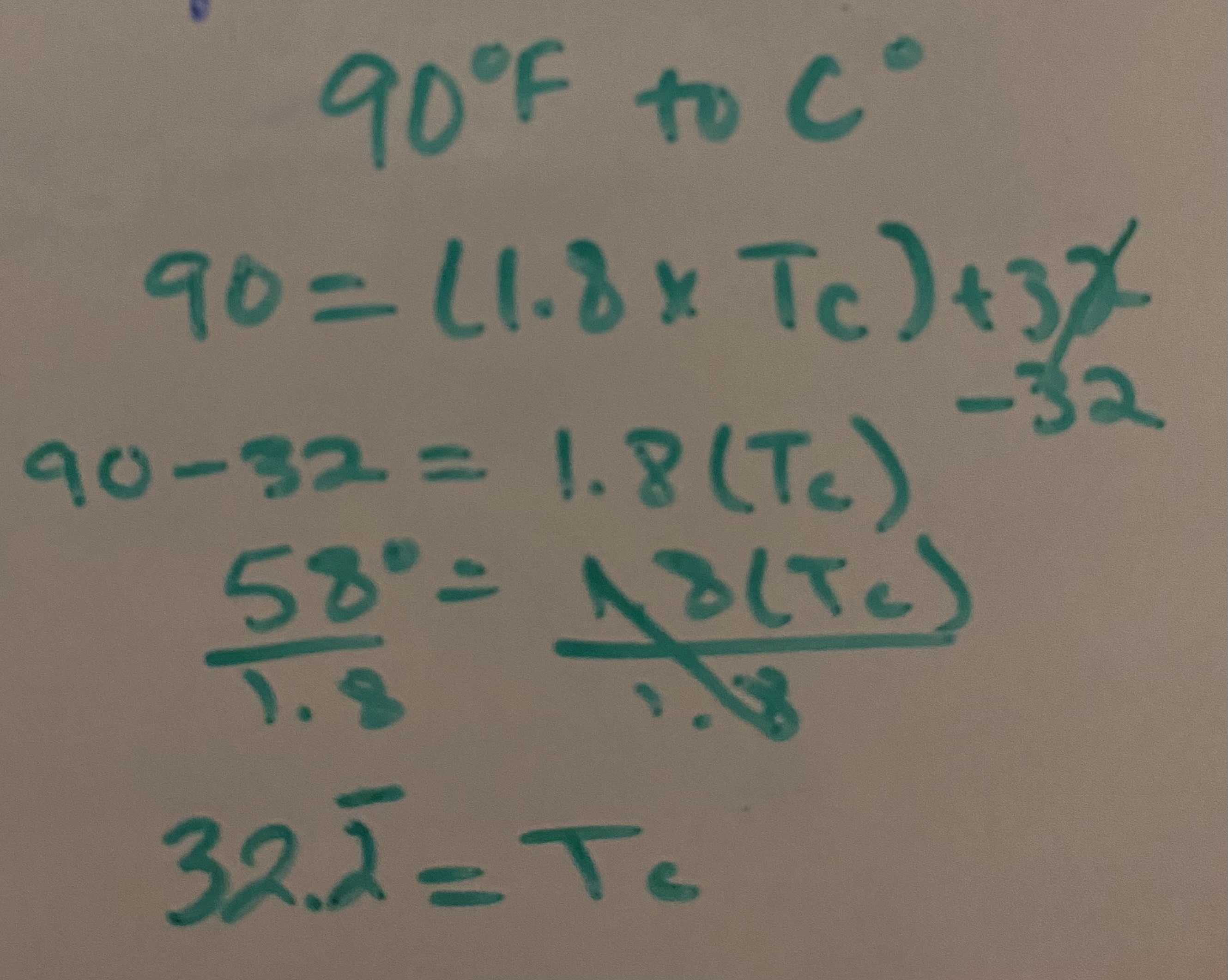
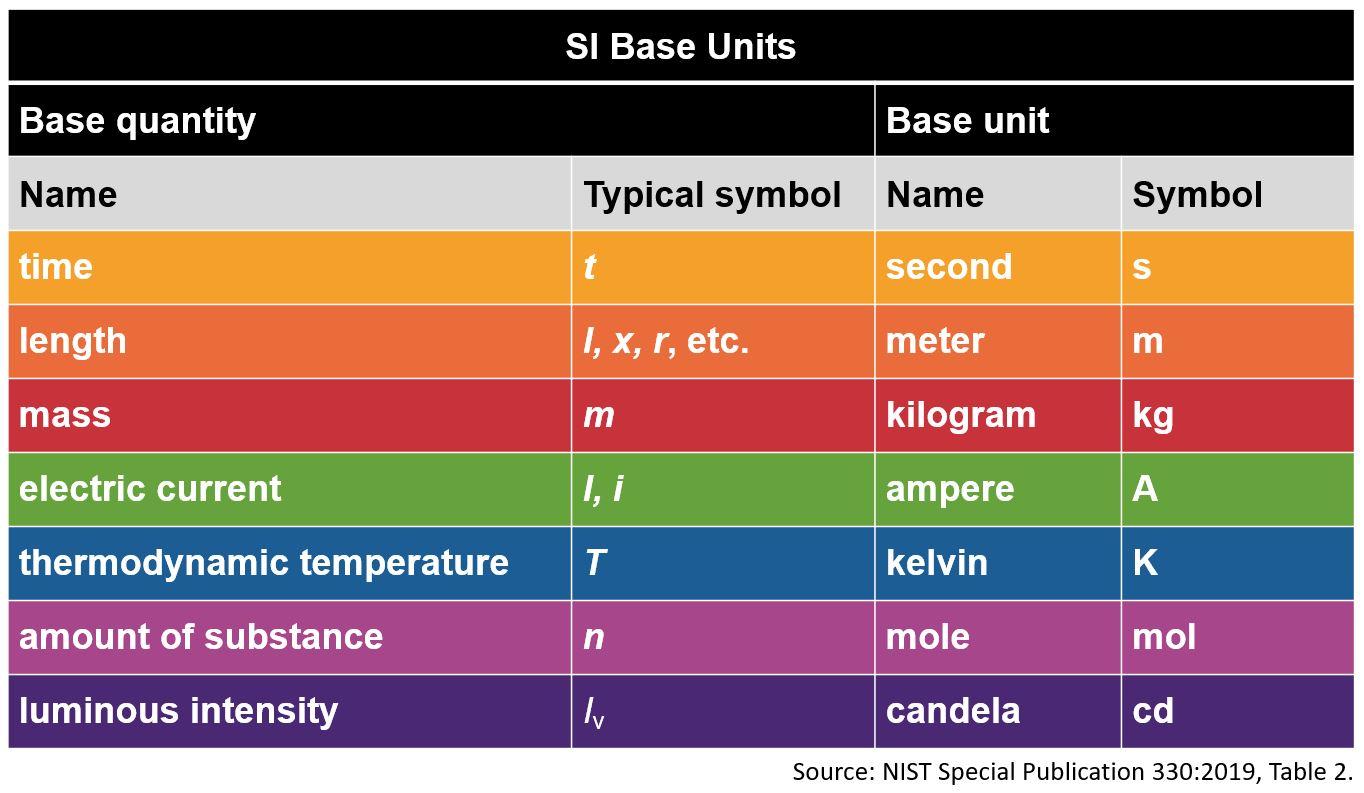
Which of the following is an SI unit?
gram
calorie
second
liter
second
Which of the following numbers is correctly expressed using scientific notation?
0.35 x 103
146.78 X 102
10.5 X10-3
2.4 X 104
2.4 X 104
Dilution of serum sample is required for a procedure. How much diluent is required to make 1 to 4 dilution with a total volume of 200μL?
The number 6073.03 contains six significant figures.
True
False
True
What is the molarity of a solution that contains 5 mol of hydrochloric acid in 2L of solution?
5M
10M
0.4M
2.5M
2.5M
What is the gram molecular weight of Cholestrol (C27H46O)?
(Atomic weight of C= 12, H= 1, O= 16)
386
Molarity (M) is calculated as:
. Mwould be considered a derived unit.
True
False
True
What is the dilution factor if 10 mL of sample is added to 190 mL of diluent?
19
20
10
25
20
Express 0.0000235 in scientific notation:
2.35 × 10-5
235 × 10-7
2.35 × 10-4
2.35 × 105
2.35 × 10-5
__________________ is a method of writing numbers in terms of decimal numbers between one and nine multiplied by a power of ten.
scientific notation
What is the normality of a solution that contains 98 gram of sulfuric acid (H2SO4) in 1L of solution? (Atomic weight H= 1, S= 32, O=16; Valance 2)
3N
6N
4N
2N
2N
How much serum is required to make a 1 to 4 dilution with a total volume of 100.0 µL?
2.5 µL
0.25 µL
0.025 µL
25 µL
25 µL
The basic unit of length in the metric system is the _____ .
foot
millimeter
mil
meter
meter
100 mL of a 1.5 M solution are diluted to 250 mL in a 250-mL volumetric flask. What is the concentration of the new solution?
3.75M
1.2M
2M
0.6M
0.6M
The laboratory result of a calcium level was reported as 10mg/dL. What is the concentration in mEq/L? (Atomic Weight of Calcium=40, Valance of calcium=2+)
5.0mEq/L, 5.0
The result of the glucose is too high for the instrument to read. The MLT performs a dilution using 20μL of patient sample to 80μL of diluent. The result now reads 150 mg/dL. What value should the technician report?
350mg/dL
150mg/dL
750mg/dL
550mg/dL
750mg/dL
Do the following calculation and express the answer using correct scientific notation.
103 / 107
10^−4 or 0.0001
How many milliliters of 6M solution would be needed to make 90mL of a 2M solution?
30 mL
Round the number 7.44 to 2 digits________.
7.4
To convert milligrams to grams, you should multiply by 1,000 g/milligram. Ture or False.
False
Round the number 8.75 to 2 digits__________________
8.8
A serum creatine kinase is diluted 1:200 with a result of 50 U/L. What is the patient's actual creatine kinase result?
10,000 U/L
200 U/L
1000 U/L
50 U/L
10,000 U/L
What is the dilution factor if 10 mL of reagent is added to 390 mL of diluent?
1 : 40
1 : 39
10 : 400
10 : 390
1 : 40
Do the following calculation and express the answer using the correct notation
(1.2 × 10 -4) × (3.0 × 10−3) = _________
3.6 × 10-1
3.6 × 10-7
3.6 × 101
3.6 × 107
3.6 × 10-7
Divide 3.1 x 10-3 by 8.4 x 10-2
3.7 x 10-5
3.7 x 10-2
2.7 x 10-5
2.7 x 10-2
3.7 x 10-2
Dilution of serum sample is required for a procedure. How much diluent is required to make 1 to 4 dilution with a total volume of 400 microliters?
300 microliter
Determine the molarity (M) of a solution if 200 mL of a 5.0 M solution is diluted to a final volume of 500 mL.
2M