Partial pressures and transport of carbon dioxide
1/15
Earn XP
Description and Tags
week 4, lesson 1, unit 5
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
where does carbon dioxide leave
leaves the blood in the pulmonary capillaries, enter the alveoli, and be exhaled out. If we want CO2 to leave the pulmonary capillaries, the PCO2 must be HIGHER inside those capillaries and LOWER inside the lungs' alveoli.
partial pressure of co2 of atmospheric air
0.3 mmHg (760 mmHg x 0.04%). So atmospheric air has low CO2relative to oxygen
resting tissue pco2
equal or greater than (if its a metabolically active tissue) 46 mmHg. so if you were active, it would be ~48/50 bc its creating more co2 in the process of creating atp
pco2 in tissues, venous and pulmonary
the same, 46 mmHg for tissue and venous bc it reaches equilibrium but its the same for pulmonary artery because no contact with capillaries
at the pulmonary capillaries, explain the movement of co2
(blood) high to low (alveolar pco2) into alveoli
alveolar pco2, pulmonary vein, arterial pco2
40 mmhg
plasma and co2
about 7% of the CO2 we carry in our blood is dissolved in plasma. this is a higher percentage than we saw for oxygen (1.5%) simply bc CO2 is more soluble in fluids than oxygen
Red blood cells (erythrocytes.)
about 23% of the co2 we carry is attached to hemoglobin and other proteins found in the blood. we call. this carbamino form of CO2 transport. but CO2 is not attached to hemegroups like we saw with oxygen. it binds to the globin chains in hemoglobin to be carried inside red blood cells
Red blood cells/plasma:
most of the carbon dioxide we carry in our blood is not carried as co2, but as an ion called bicarbonate (HCO3) this rxn takes place inside red blood cells bc the rxn requires an enzyme that is found in the cytoplasm of red blood cells. this enzyme is called carbonic anhydrase. when bicarbonate is made thru this rxn, we also make hydrogen ion (proton)
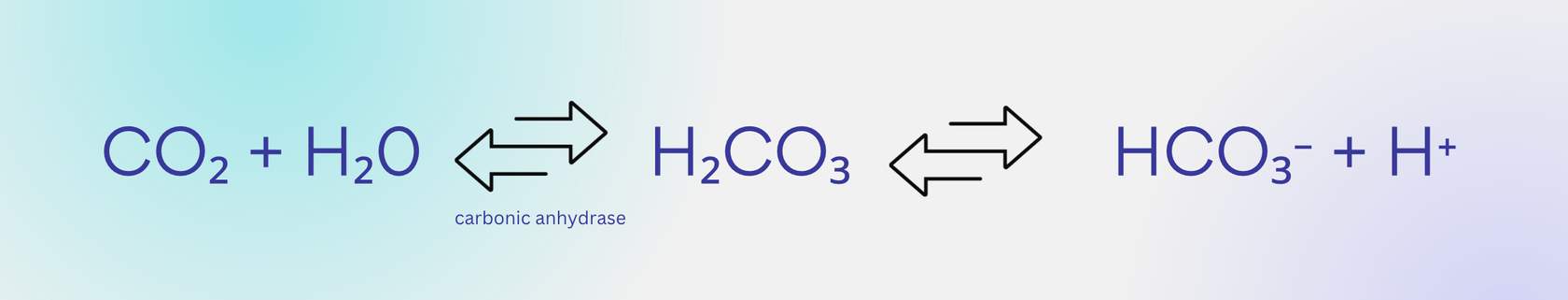
When CO2 inside the red blood cells reacts with water
inside the cytoplasm of the red blood cell in the presence of carbonic anhydrase, it initially creates carbonic acid (H2CO3). However, carbonic acid is pretty unstable, so it quickly dissociates (breaks down) into bicarbonate and hydrogen. This entire chemical reaction is reversible. Like any chemical reaction, the direction of the reaction depends on how much of each substance is on either side of the equation. So when there is lots of CO2 entering the blood, the reaction goes to the right and creates lots of bicarbonate.
Once bicarbonate (HCO3) forms,
it leaves the red blood cell through a transporter found in that red blood cell's plasma membrane and is carried around in plasma. Why? Well, if we didn't remove HCO3-, then we would eventually build up too muchHCO3- inside of the cell and that would shift the equation back to the left. But we want to keep picking up CO2 at the tissue, so we want to make sure HCO3- levels remain low inside red blood cells.
At the lung alveoli and bicarbonate levels
At the lung alveoli, we want to get rid of CO2. This requires the bicarbonate levels to be higher than CO2 levels to drive the reaction in reverse and shift it to the left, changing bicarbonate back into CO2. That same transporter must bring bicarbonate back into the red blood cell to make this happen, since carbonic anhydrase is required for that reaction.
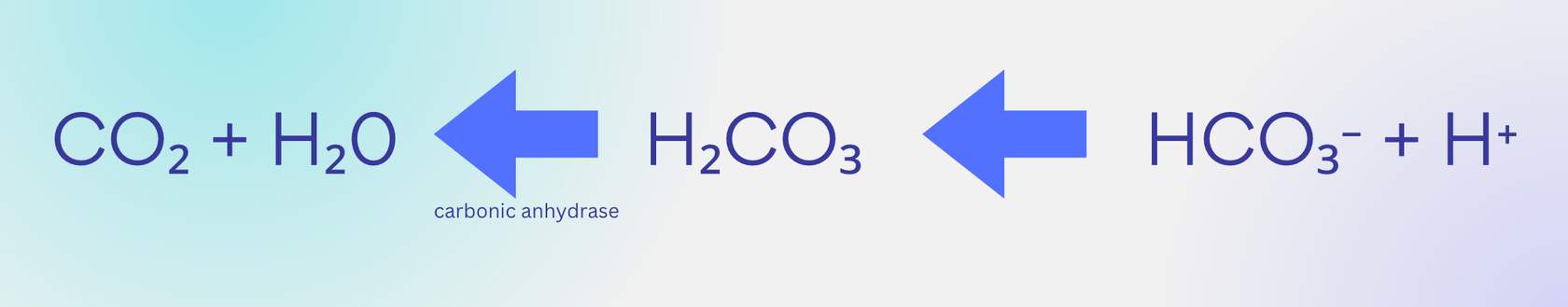
Pulmonary capillaries: unloading CO2
If you remember the goal is to pick up CO2 at the tissues and return it to the lungs to be exhaled out, the direction of this equation should make sense. We want to "free up" CO2 that was carried as bicarbonate and turn it into a gas again so it can enter alveolar air.
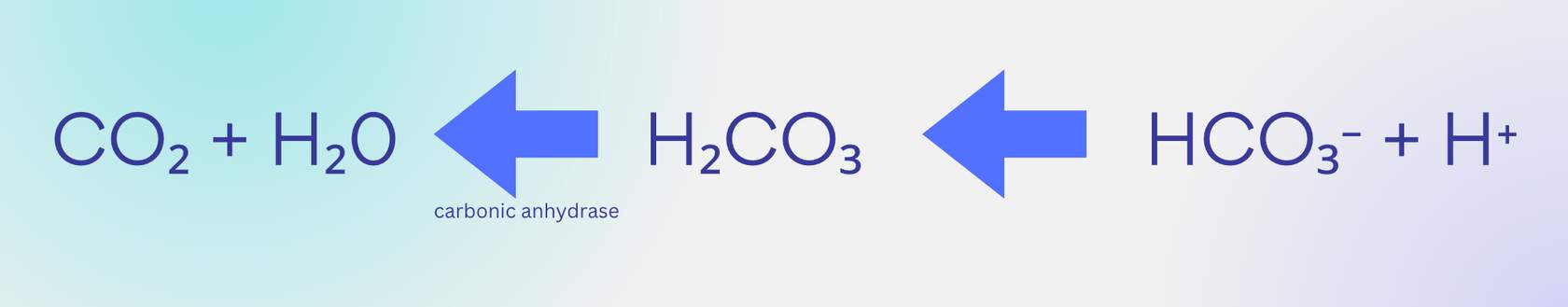
As some CO2 leaves the blood at lung capillaries, this creates an imbalance
with more bicarbonate than CO2, causing the equation to shift to the left. Therefore, blood leaving the lung capillaries has less bicarbonate as a result. It also has less H+, which you will see is related to the pH of our blood. H+ is acidic, so the blood leaving our lungs has a higher pH (more basic) than the blood entering our lungs because it has less H+.
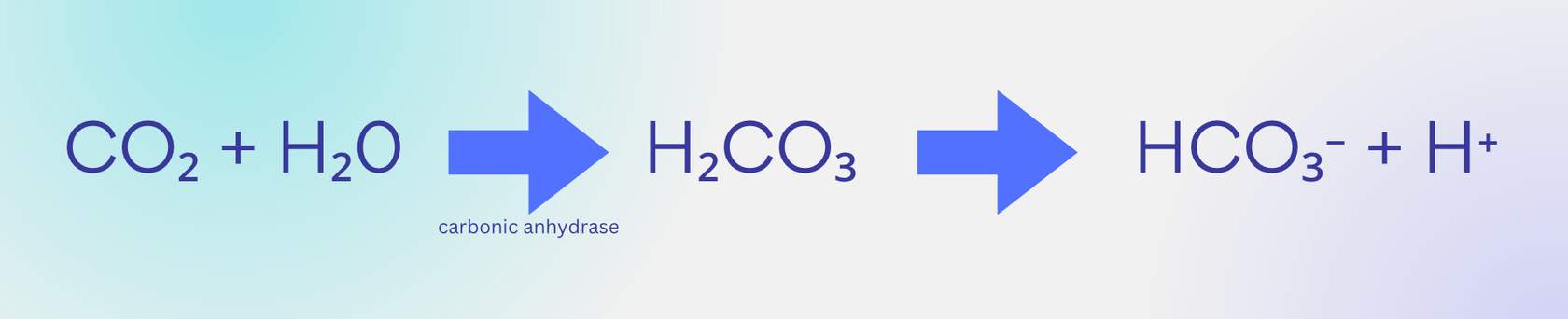
Loading up CO2 in the blood: systemic capillaries
At the tissues, we want to load CO2 into systemic capillaries and carry it through the blood, mainly in the form of bicarbonate (HCO3-). So, since CO2 is abundant in these capillaries, it drives the reaction inside red blood cells to the right. But also notice that this creates a lot of hydrogen (H+) as well, which is acidic. This means that the blood in our systemic veins leaving the tissue has a lower pH (more acidic).
bicarbonate transported out of the red blood cell
bicarbonate gets out, cl comes in. both are negatively charged ions so it stays electrically neutral