Atomic Structure and Electron Structure
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
1
New cards
Relative Atomic Mass
Average mass of one atom of an element compared with 1/12th mass of an atom of Carbon-12
2
New cards
RAM
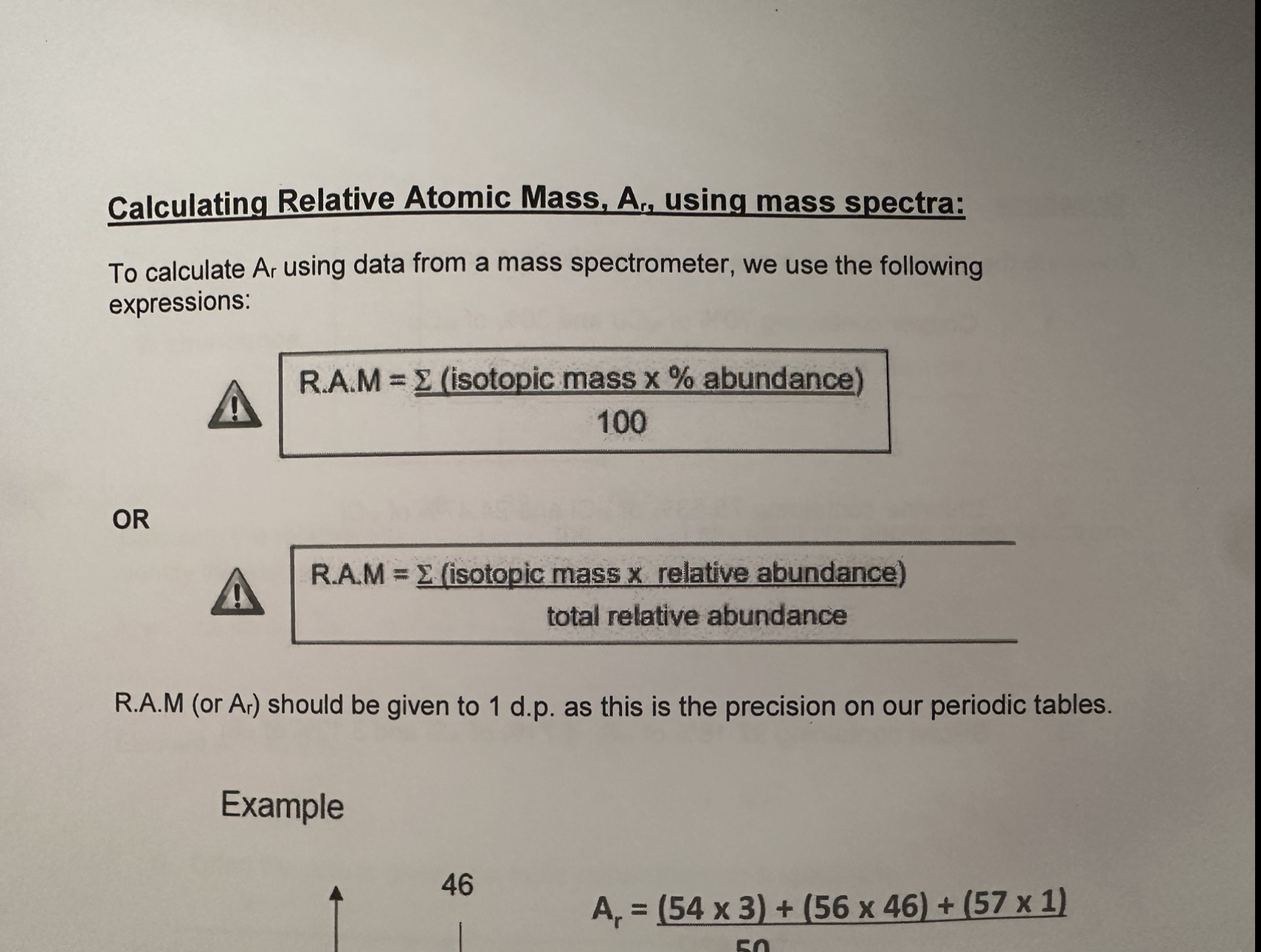
3
New cards
An Atomic Orbital
A region within an atom that can hold up to 2 electrons with opposite spins
4
New cards
S and P oribtals
S orbital- spherical
P orbital - dumbell shape
5
New cards
Electron Configuration
1s22s22p63s23p64s23d104p65s2
Shorthand configuration can be written using the noble gas configuration that comes before the element e.g.
Si: 1s22s22p63s23p2→ [Ne] 3s23p2
6
New cards
Electron Configuration Exceptions and Reasons
Chromium: 1s22s22p63s23p64s13d5
Copper: 1s22s22p63s23p64s13d10
Reasons: These represents more stable arrangements