Physics chapters 2,3 and 4
5.0(2)
Card Sorting
1/118
Earn XP
Last updated 1:45 AM on 4/20/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
119 Terms
1
New cards
Radiography as art
knowing how to think outside the box
2
New cards
Radiography as science
* Physical science: nonliving matter
* Physics
* Biological science: living matter
* Anatomy and physiology
* Physics
* Biological science: living matter
* Anatomy and physiology
3
New cards
Physics studies relationships between matter and energy
* matter has mass and occupies space
* Energy is force used to do work
* unit of energy is joule (J)
* Energy is force used to do work
* unit of energy is joule (J)
4
New cards
Law of conservation of matter and energy
* Matter and energy cannot be destroyed, just changed
* Matter and energy have a unique relationship
* Sum of all matter and energy is constant
* Theorized by Albert Einstein in 1905
* E=mc^2
* Matter and energy have a unique relationship
* Sum of all matter and energy is constant
* Theorized by Albert Einstein in 1905
* E=mc^2
5
New cards
Structure of matter
* Substances
* Simple versus complex
* Elements versus compounds
* Atoms versus molecules
* Mixtures
* Two or more substances combined
* Simple versus complex
* Elements versus compounds
* Atoms versus molecules
* Mixtures
* Two or more substances combined
6
New cards
Simple versus complex
* Simple
* Element
> Atom
* Complex
* Compound
> Molecule
* Element
> Atom
* Complex
* Compound
> Molecule
7
New cards
Compound
2 or more elements chemically combined
8
New cards
Molecule
2 or more atoms chemically combined
9
New cards
Molecules
* Smallest particle of a compound possessing characteristics of the compound
* Consist of two or more atoms that are chemically united
* Held together by chemical bonds
* Consist of two or more atoms that are chemically united
* Held together by chemical bonds
10
New cards
Laws of attraction
* Atoms are bound to one another by varying degrees of attraction
* States: Solid, liquid, gas
* States: Solid, liquid, gas
11
New cards
The degree of attraction between the atom is what determines the state of the matter
* Solid: atoms that are tightly bond together
* Gas: atoms that are loosely bond together
* Liquid: in between a solid and gas
* Gas: atoms that are loosely bond together
* Liquid: in between a solid and gas
12
New cards
Ancient greeks found matter was made of 4 components
Air, water, earth, and fire
13
New cards
Dalton (early 1800s)
* Separated elements based on mass
* Lead to Mendeleev’s development of periodic table of elements
* Lead to Mendeleev’s development of periodic table of elements
14
New cards
Rutherford (1911)
Created a model of the atom that has a positive nucleus surrounded by cloud of negative electrons
15
New cards
Bohr (1913)
Expanded on Rutherfords model creating a model of atom likened to a miniature solar system
16
New cards
Schrödinger
Foundation of modern physics (quantum physics or wave mechanics)
17
New cards
Atomic structure
* Atom is made of 3 parts
* Protons, neutrons, and electrons
* Nucleus
* Small, dense center
* Contains nucleons, protons, and neutron
* Electrons
* Orbit nucleus in a variety of planes
* In specific energy states
* Cannot be divided into smaller parts
* All of x-ray magic start at the atomic level
* Protons, neutrons, and electrons
* Nucleus
* Small, dense center
* Contains nucleons, protons, and neutron
* Electrons
* Orbit nucleus in a variety of planes
* In specific energy states
* Cannot be divided into smaller parts
* All of x-ray magic start at the atomic level
18
New cards
Basic atomic particles
* Subatomic particles (each have their own charge)
* Proton
* Positive charge; Mass number 1
* Neutron
* Neutral charge/no charge; Mass number 1
* Electron
* Negative charge; Very small mass (essentially 0 mass)
* Proton
* Positive charge; Mass number 1
* Neutron
* Neutral charge/no charge; Mass number 1
* Electron
* Negative charge; Very small mass (essentially 0 mass)
19
New cards
Atom
* Neutral or electrical stability
* When maintained through equal number of protons and electrons
* Atomic number (Z#)
* Distinguishes elements by number of protons contained in nucleus
* Examples:
* Hydrogen has 1 proton and its atomic number is 1
* Lead has 82 protons and its atomic number is 82
* When maintained through equal number of protons and electrons
* Atomic number (Z#)
* Distinguishes elements by number of protons contained in nucleus
* Examples:
* Hydrogen has 1 proton and its atomic number is 1
* Lead has 82 protons and its atomic number is 82
20
New cards
Changing elements
* Having an element changed from one element to another does not happen commonly because its not a natural occurrence, the only way to have an element changed is by
* Changing the protons or change the Z#
> This process happens during radioactive decay
* Example: Radioactive decay
> Radium (Z# 88) emits alpha particle and decays to Radon (Z# 86)
* Changing the protons or change the Z#
> This process happens during radioactive decay
* Example: Radioactive decay
> Radium (Z# 88) emits alpha particle and decays to Radon (Z# 86)
21
New cards
Other atomic variations
* Changing the number of neutrons or electrons does not change the element
* Isotopes - if an atom gains or loses neutrons
* Ions - if an atom gains or loses electrons
> Ionization is the process of adding or removing electrons from an atom
* Changing the number of neutrons, changes atomic mass only
* Isotopes - if an atom gains or loses neutrons
* Ions - if an atom gains or loses electrons
> Ionization is the process of adding or removing electrons from an atom
* Changing the number of neutrons, changes atomic mass only
22
New cards
Isotopes
an atom gains or loses neutrons
23
New cards
Ions
an atom gains or loses electrons
24
New cards
Ionization
the process of adding or removing electrons from an atom
25
New cards
More on ionization
* Addition or removal of electron from atom
* X-ray photons can interact with atom within the human body
* Results in ejection of electron
* Changes charges between atoms
* Ionization of atoms causes disruptions in body’s metabolic relationships
* Exposing patients to ionizing radiation can produce short-term and long-term effects therefore x-rays requires order from a licensed practitioner
* X-ray photons can interact with atom within the human body
* Results in ejection of electron
* Changes charges between atoms
* Ionization of atoms causes disruptions in body’s metabolic relationships
* Exposing patients to ionizing radiation can produce short-term and long-term effects therefore x-rays requires order from a licensed practitioner
26
New cards
Atomic mass
* Concentrated in nucleus (this is because electrons have very little mass)
* Mass of proton 1,836 times greater than electron (proton mass #: 1)
* Mass of neutron 1,838 times greater than electron (neutron mass #: 1)
* Atomic mass number (A#)
* Consists of total protons and neutrons
* Neglects mass of atom’s electrons
* Mass of proton 1,836 times greater than electron (proton mass #: 1)
* Mass of neutron 1,838 times greater than electron (neutron mass #: 1)
* Atomic mass number (A#)
* Consists of total protons and neutrons
* Neglects mass of atom’s electrons
27
New cards
Orbital electrons
* All electrons surrounding the nucleus are constantly in motion, they inhabit what is known as shells
* Orbital
* Defines location where electron might be at any given time in atom
* Shells: labeled by letters or numbers
* Formerly: K, L, M, N, O, P, Q (slowly fading away for method of labeling)
* Now: 1, 2, 3, 4, 5, 6, 7
* Electron capacity = 2n^2 : maximum number of electrons per shell
* n = the orbital shell number
* Shell 7 = 2(7)^2 = 98
* Orbital
* Defines location where electron might be at any given time in atom
* Shells: labeled by letters or numbers
* Formerly: K, L, M, N, O, P, Q (slowly fading away for method of labeling)
* Now: 1, 2, 3, 4, 5, 6, 7
* Electron capacity = 2n^2 : maximum number of electrons per shell
* n = the orbital shell number
* Shell 7 = 2(7)^2 = 98
28
New cards
Electron binding energy (Eb)
* Energy needed to eject electron from atom (ionization)
* Related to how close electron is to nucleus
> The closer the electron is from the nucleus, the harder it is to eject
* The k shell is closest to nucleus so it will be the hardest to eject from atom it
* The p shell is the farthest so it will be easier to eject electrons from that shell
* Eb increases as Z# increases
* Related to how close electron is to nucleus
> The closer the electron is from the nucleus, the harder it is to eject
* The k shell is closest to nucleus so it will be the hardest to eject from atom it
* The p shell is the farthest so it will be easier to eject electrons from that shell
* Eb increases as Z# increases
29
New cards
Electron binding energy (Eb)
* Determined by two primary factors
* Electron proximity to nucleus
* Number of protons in nucleus
* Unit: electron volts (eV)
* Energy of one electron when accelerated by one volt
* Understanding the concept of electron binding energy is a key concept in radiology physics because x-rays are capable of ionizing atoms through radiation
* Hydrogen, carbon, & oxygen are primary component of human body
* Iodine & barium are used as contrast media
* Tungsten is used in x-ray tube
* Lead is used for radiation protection
* Electron proximity to nucleus
* Number of protons in nucleus
* Unit: electron volts (eV)
* Energy of one electron when accelerated by one volt
* Understanding the concept of electron binding energy is a key concept in radiology physics because x-rays are capable of ionizing atoms through radiation
* Hydrogen, carbon, & oxygen are primary component of human body
* Iodine & barium are used as contrast media
* Tungsten is used in x-ray tube
* Lead is used for radiation protection
30
New cards
Elements that can be ionized by radiation
Hydrogen, Carbon, Oxygen, Iodine, Barium, Tungsten, and Lead
31
New cards
Periodic table of elements
* Elements are arranged into horizontal periods & vertical groups
* Horizontal periods represent elements with the same # of electron shells
* Vertical groups (columns) represent elements with the same # of electrons in the outer most shell
* Elements are listed in ascending order based on the atomic #
* Not every element has a symbol that related to its name
* Atomic number represents the number or protons in the element
* Horizontal periods represent elements with the same # of electron shells
* Vertical groups (columns) represent elements with the same # of electrons in the outer most shell
* Elements are listed in ascending order based on the atomic #
* Not every element has a symbol that related to its name
* Atomic number represents the number or protons in the element
32
New cards
Valence
* The # of electrons in the outer shell will determine if it gives up electrons or gains electrons
* If elements have 4 or less valence electrons, it will lose electrons
* If elements have more than 4 valence electrons, it will gain electrons
* If elements have 4 or less valence electrons, it will lose electrons
* If elements have more than 4 valence electrons, it will gain electrons
33
New cards
Valence definition
Chemical behavior of the element is determined by how many electrons are in the outer most shell of an element
34
New cards
Octet rule
Atoms want to abide to this rule which states atoms want to obtain 8 electrons in the outer shell
35
New cards
Work defined
* Definition of energy
* Ability to do work
* Physicist’s definition of work
* Work = force X distance
* When a force acts upon an object over a distance, energy is expended. This is work.
* Ability to do work
* Physicist’s definition of work
* Work = force X distance
* When a force acts upon an object over a distance, energy is expended. This is work.
36
New cards
6 types of energy
* Mechanical
* Chemical
* Heat
* Electrical
* Nuclear
* Electromagnetic
* Chemical
* Heat
* Electrical
* Nuclear
* Electromagnetic
37
New cards
Mechanical energy
* The action of physical movement by either machine or by human
* 2 types: Potential and Kinetic energy
* Potential energy: energy that an object has because of its position, object might be at rest but its ready to move
* Kinetic energy: the energy of motion, an object in motion can perform work because it is moving
* 2 types: Potential and Kinetic energy
* Potential energy: energy that an object has because of its position, object might be at rest but its ready to move
* Kinetic energy: the energy of motion, an object in motion can perform work because it is moving
38
New cards
Chemical energy
* The energy released from chemical reactions
* EX: battery converting chemical energy into electrical energy
* EX: body converting chemical energy from food into mechanical energy (movement)
* EX: battery converting chemical energy into electrical energy
* EX: body converting chemical energy from food into mechanical energy (movement)
39
New cards
Heat energy AKA thermal energy
* The result of moving atoms & molecules
* The amount of thermal energy created is dependent on how fast the atoms or molecules move or vibrate within a substance
* The faster they move, the ore thermal energy it produces
* X-ray production produces high thermal energy
* Temperature is the measure of thermal energy and can be measured in Fahrenheit or Celsius
* The amount of thermal energy created is dependent on how fast the atoms or molecules move or vibrate within a substance
* The faster they move, the ore thermal energy it produces
* X-ray production produces high thermal energy
* Temperature is the measure of thermal energy and can be measured in Fahrenheit or Celsius
40
New cards
Electrical energy AKA electricity
* Results from the movement of electrons in a conductor
* EX: lightbulb is a device that converts electric energy into light
* EX: lightbulb is a device that converts electric energy into light
41
New cards
Nuclear energy
* Obtained by breaking the bonds between particles within the nucleus
* Protons & neutrons inhabit the nucleus
* EX: nuclear power-plants convert nuclear energy into thermal energy that is converted into electricity
* Protons & neutrons inhabit the nucleus
* EX: nuclear power-plants convert nuclear energy into thermal energy that is converted into electricity
42
New cards
Electromagnetic (EM) energy
* Results from electric & magnetic disturbances in space
* Travels as waves and has many familiar forms such as microwaves, radio-waves, cosmic waves and x-rays
* Travels as waves and has many familiar forms such as microwaves, radio-waves, cosmic waves and x-rays
43
New cards
Electromagnetic (EM) radiation AKA electromagnetic energy (same definition)
* The electromagnetic spectrum
* Covers all forms of EM radiation
* Something that is common to all forms of EM radiation is that their velocity is equal to the speed of light in a vacuum
* EM energy is capable of ionizing an atom or molecule when it is measured @ 10 eV or hight
* EM radiation is considered to have wave particle duality, this means sometimes an x-ray photon behaves as a wave and sometimes it behaves as a particle
* Covers all forms of EM radiation
* Something that is common to all forms of EM radiation is that their velocity is equal to the speed of light in a vacuum
* EM energy is capable of ionizing an atom or molecule when it is measured @ 10 eV or hight
* EM radiation is considered to have wave particle duality, this means sometimes an x-ray photon behaves as a wave and sometimes it behaves as a particle
44
New cards
Characteristics of EM Radiation
* EM energy travels through space in the forms of waves and these waves create a disturbance
* 3 characteristics of the wave
* Wavelength
* Frequency
* Amplitude (energy)
* 3 characteristics of the wave
* Wavelength
* Frequency
* Amplitude (energy)
45
New cards
Wavelength
* The distance between any 2 identical points on a wave within a cycle.
* Measured from crest to crest or trough to trough
* Crest: top of wave
* Trough: bottom of wave
* Represented by greek letter (symbol) Lambda
* Measured from crest to crest or trough to trough
* Crest: top of wave
* Trough: bottom of wave
* Represented by greek letter (symbol) Lambda

46
New cards
Frequency
* The number of times a wave passes a particular point in a given time frame or the number of cycles per second
* Unit of frequency is hertz
* Represented by greek letter (symbol) is nu
* Frequency and wavelength are inversely proportional
* That means that short wavelengths will have high frequency and long wavelengths will have low frequency
* Unit of frequency is hertz
* Represented by greek letter (symbol) is nu
* Frequency and wavelength are inversely proportional
* That means that short wavelengths will have high frequency and long wavelengths will have low frequency

47
New cards
Amplitude (energy)
* The intensity or energy (amplitude) Celoof the wave is defined by its maximum height
* No greek letter (symbol)
* No greek letter (symbol)
48
New cards
Velocity, Wavelength, and Frequency
* A relationship exists between velocity, wavelength, and frequency
* Velocity: in m/s
* Wavelength: in m
* Frequency: in Hz or 1/s
* Expressed with the formula: V= Wavelength X Frequency (the picture)
* Velocity: in m/s
* Wavelength: in m
* Frequency: in Hz or 1/s
* Expressed with the formula: V= Wavelength X Frequency (the picture)
49
New cards
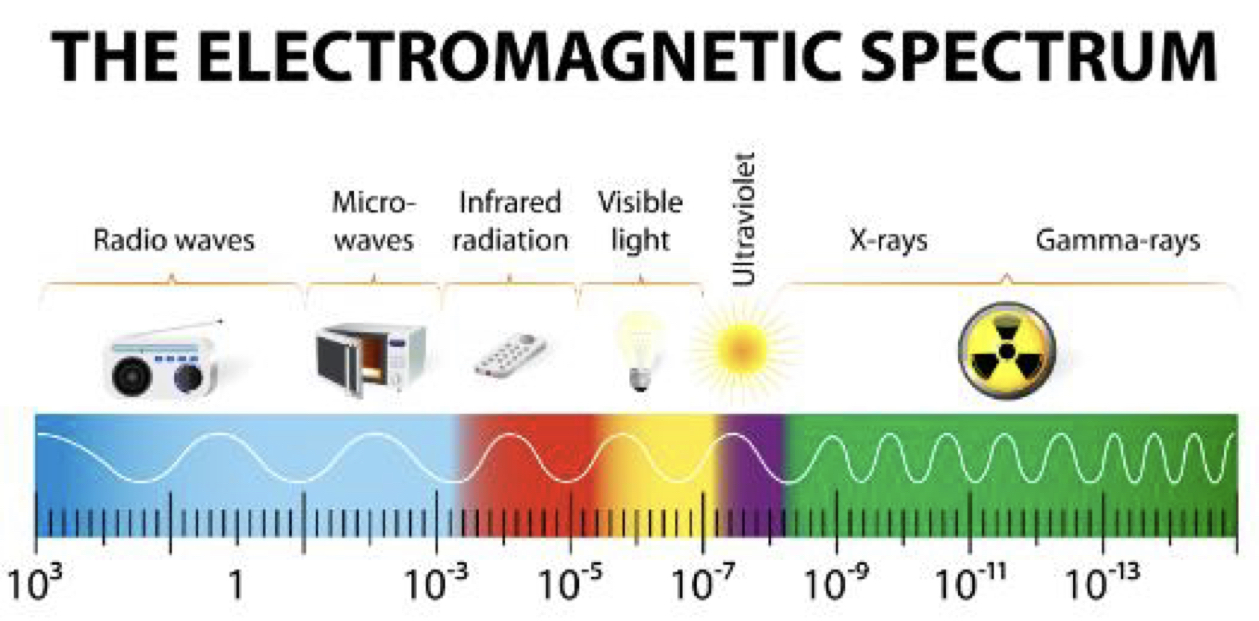
Important thing to know
it is important to know the inverse relationship between wavelength and frequency (inversely proportional) and to know that low frequency wavelengths are at the bottom of the spectrum (radio-waves & microwaves) and high frequency wavelengths are at the top of the spectrum (x-rays)
50
New cards
Particle Theory states
* High frequency, high energy EM radiation
* Behaves more like particle when contacting matter
* Photons energy and frequency directly related (directly proportional)
* If frequency doubles, energy doubles
* If one goes up, the other goes up
* Behaves more like particle when contacting matter
* Photons energy and frequency directly related (directly proportional)
* If frequency doubles, energy doubles
* If one goes up, the other goes up
51
New cards
Discovery of x-rays
* Wilhelm Conrad Roentgen discovered x-rays by accident
* Awarded first noble prize for physics due to discovery
* Wilhelm Conrad Roentgen is the “RAD FATHER”
* Awarded first noble prize for physics due to discovery
* Wilhelm Conrad Roentgen is the “RAD FATHER”
52
New cards
X-ray properties
1. X-rays are highly penetrating, invisible rays that are a form of EM radiation
2. X-rays are electrically neutral, therefore cannot be affected by electric or magnetic fields
3. X-rays produce over a wide variety of energies & wavelengths
4. X-rays release very small amount of heat upon passing through matter
5. X-rays travels in straight lines
6. X-rays travel at speed of light (3X10^8 m/s in a vacuum)
53
New cards
X-ray properties continued
7. X-rays can ionize matter
8. X-rays can cause fluorescence in certain crystals
9. X-rays cannot be focused by lens
10. X-rays can affect photographic film
11. X-rays can produce chemical and biological changes in matter through ionization and excitation
12. X-rays can produce secondary and scatter radiation
54
New cards
Atomic Nature of electricity
* Electricity concerns the distribution and movement of electrons
* Protons - confined to nucleus
* Smallest unit of positive charge
* Locked within the nucleus
* Electrons - Free to move between orbital shells within atom or even more to another atom
* Smallest unit of negative charge
* Free to move between orbitals and atoms
* Protons - confined to nucleus
* Smallest unit of positive charge
* Locked within the nucleus
* Electrons - Free to move between orbital shells within atom or even more to another atom
* Smallest unit of negative charge
* Free to move between orbitals and atoms
55
New cards
Electrostatics
* The study of the distribution of fixed charges
* Defined as fixed charges or electrons at rest
* When we study electrostatics, we study the distribution & redistribution of charged electrons
* Electrification - electron charge being added or subtracted from another object (negative or positive)
* When one object has more electrons than another object, its said to be negatively electrified
* Positive electrification is in reference to something with a weak or negative charge or fewer electrons than the object its being compared too
* When discussing electricity, the terms negative and positive refer to the relationship between two objects, not their true atomic charges
* Defined as fixed charges or electrons at rest
* When we study electrostatics, we study the distribution & redistribution of charged electrons
* Electrification - electron charge being added or subtracted from another object (negative or positive)
* When one object has more electrons than another object, its said to be negatively electrified
* Positive electrification is in reference to something with a weak or negative charge or fewer electrons than the object its being compared too
* When discussing electricity, the terms negative and positive refer to the relationship between two objects, not their true atomic charges
56
New cards
Laws of electrostatics
* Law of repulsion-attraction
* Inverse square law
* Law of distribution
* Law of concentration
* Law of movement
* Inverse square law
* Law of distribution
* Law of concentration
* Law of movement
57
New cards
Law of repulsion-attraction
This law states that opposite charges attract and like charges repel
58
New cards
Inverse square law
* The force between two charges is directly proportional to the product of their magnitudes and inversely proportional to the square of distance between them
* In other words…. as a charges object gets further away the influencing charge decreases because of the increased area it affects
* Good ex: Turn flashlight on and stand 6 in from a wall, the area that the light covers is small and the light is bright. Slowly walk backwards, as the flashlight gets further away from the wall, the small dot grows, the area gets larger and light gets dimmer
* In other words…. as a charges object gets further away the influencing charge decreases because of the increased area it affects
* Good ex: Turn flashlight on and stand 6 in from a wall, the area that the light covers is small and the light is bright. Slowly walk backwards, as the flashlight gets further away from the wall, the small dot grows, the area gets larger and light gets dimmer

59
New cards
Law of distribution
* Charges reside on external surface of solid conductors
* This is the result of the repulsion attraction law
* Electrons all negatively charges attempt to repel each other as much as possible and the result is that they spread out creating equal distribution
* Charges attempt to repel each other due to negative charges resulting in equal distribution
* In non-conductors charges are equally distributed throughout the entire object
* This is the result of the repulsion attraction law
* Electrons all negatively charges attempt to repel each other as much as possible and the result is that they spread out creating equal distribution
* Charges attempt to repel each other due to negative charges resulting in equal distribution
* In non-conductors charges are equally distributed throughout the entire object
60
New cards
Law of concentration
* Greater concentration of charge will gather at sharpest area of curvature
* On a cylindrical wire, charges are equidistant from each other
* The goal is to have equal distribution among the electrons, if too many electrons gather together, they can start to ionize the error around them and things can become unstable
* To avoid out-of-control electrons the inferior components of the x-ray tube have parts that are rounded and polished to avoid sharp curves therefore avoiding the gathering of too many electrons
* On a cylindrical wire, charges are equidistant from each other
* The goal is to have equal distribution among the electrons, if too many electrons gather together, they can start to ionize the error around them and things can become unstable
* To avoid out-of-control electrons the inferior components of the x-ray tube have parts that are rounded and polished to avoid sharp curves therefore avoiding the gathering of too many electrons
61
New cards
Law of movement
This law states that only negative charges can move along solid conductors. This is because protons are confined to the nucleus and not able to move
62
New cards
Three methods of electrification
* Friction
* Abrasion of two conductive materials creates a transfer of charges between. the materials
* Contact
* Physically touching a charged body to a neutral body, creates a transfer of charge
* Induction
* Bring a charged body in close proximity to a neutral object, without physical contact, creates a charge in the neutral object
* Abrasion of two conductive materials creates a transfer of charges between. the materials
* Contact
* Physically touching a charged body to a neutral body, creates a transfer of charge
* Induction
* Bring a charged body in close proximity to a neutral object, without physical contact, creates a charge in the neutral object
63
New cards
Electrification is the
Process of electron charges being added or subtracted from an object
64
New cards
Friction
* the resistance that one surface or object encounters when moving over another
* Friction occurs when object is rubbed against another and electrons from one object transfer to the other object
* This transfer will only take place if each of the objects have a different number of electrons
* The object have to move against one another
* This principal is better understood when thinking about balloons and hair (opposites attract)
* Friction occurs when object is rubbed against another and electrons from one object transfer to the other object
* This transfer will only take place if each of the objects have a different number of electrons
* The object have to move against one another
* This principal is better understood when thinking about balloons and hair (opposites attract)
65
New cards
Contact
* Electrification by contact occurs when 2 objects touch, permitting electrons to move from one object to another. This process equalizes the charges leaving both objects w/ similar charges after contact
* When you drag your feet across the floor, you’re gathering electrons and objects that are neutral like another person or door handle, the contact will cause the electrons to try to transfer to equally distribute themselves. When electrons do transfer its known as static discharge
* When you drag your feet across the floor, you’re gathering electrons and objects that are neutral like another person or door handle, the contact will cause the electrons to try to transfer to equally distribute themselves. When electrons do transfer its known as static discharge
66
New cards
Induction
* the process of electrical fields acting on one another w/o contact
* when a strongly charged object and a weakly charged object came close to one another their electrical fields will begin to act on one another before they even touch
- When objects are removed from one another before contact, the charges in the weaker charged object redistribute themselves, this temporarily induced movement of charges is what provide energy to electronic devices
* Induction is the method used in the operation of electronic devices
* Lighting is produced through electrification induction
* An electrical field is essentially a force field that surrounds an electrically charges particle and exerts force on all other charged particles that come into its field either attracting or repelling them.
* when a strongly charged object and a weakly charged object came close to one another their electrical fields will begin to act on one another before they even touch
- When objects are removed from one another before contact, the charges in the weaker charged object redistribute themselves, this temporarily induced movement of charges is what provide energy to electronic devices
* Induction is the method used in the operation of electronic devices
* Lighting is produced through electrification induction
* An electrical field is essentially a force field that surrounds an electrically charges particle and exerts force on all other charged particles that come into its field either attracting or repelling them.
67
New cards
Electrodynamics
* Movement of electricity
* Electric current is defined as electrons that are predominantly in the same direction, there are certain things that can encourage electrons to move together
1. a vacuum - space from which air has been removed
* When electrons are not bumping into air molecules, they reach high speeds. this is very important in x-ray production
2. Gases and ionic solutions can cause electrons to migrate
3. conductors
* Electric current is defined as electrons that are predominantly in the same direction, there are certain things that can encourage electrons to move together
1. a vacuum - space from which air has been removed
* When electrons are not bumping into air molecules, they reach high speeds. this is very important in x-ray production
2. Gases and ionic solutions can cause electrons to migrate
3. conductors
68
New cards
Conductors
Materials that easily permit electrons to flow
* EX: copper and aluminum
* EX: copper and aluminum
69
New cards
Insulators
Materials that inhibit electrons from moving
* EX: Plastic, rubber, glass
* EX: Plastic, rubber, glass
70
New cards
semiconductors
Materials that have the ability to conduct under some conditions and insulate under others
71
New cards
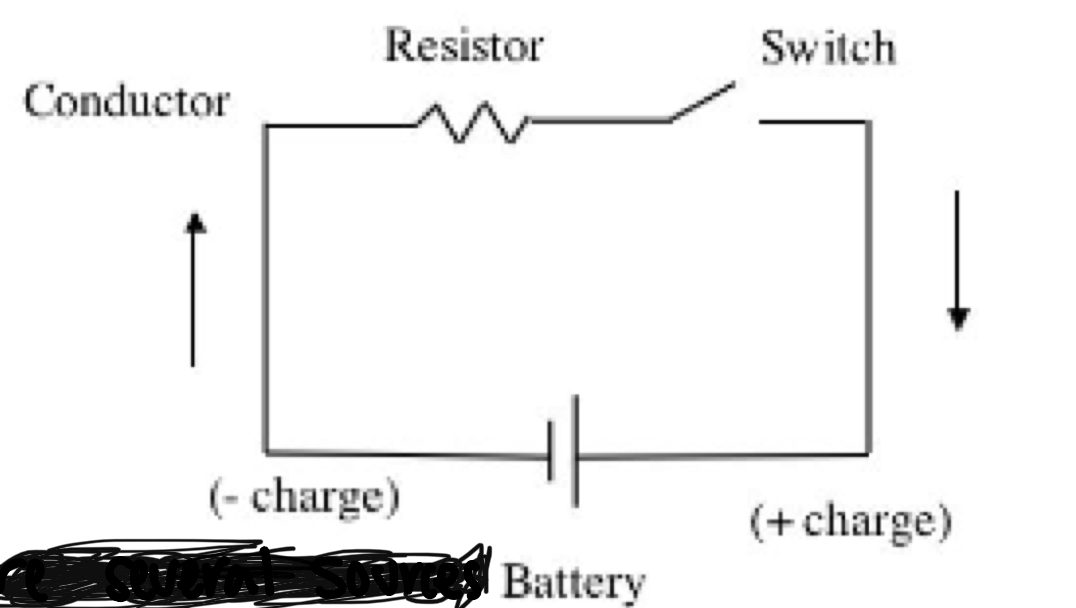
Electrical circuits
* Must have a high charge @ one end and a lower change @ the other end to allow the electrons to move
* A basic electrical circuit is a pathway that permits electrons to move in a complete circle from their source through a resisting electrical device and back to the OG source
* There are several sources that can assist electrons in moving such as batteries and generators
* X-ray tubes get their power from generators
* A basic electrical circuit is a pathway that permits electrons to move in a complete circle from their source through a resisting electrical device and back to the OG source
* There are several sources that can assist electrons in moving such as batteries and generators
* X-ray tubes get their power from generators
72
New cards
Factors of flow
* electrons move from the highest concentration of charge to the lowest
* Conventional electric current is described as going from positive to negatives poles
* Electron flow is the movement from negative poles to positive poles
* Quantity of electrons flowing
* Also known as current
* Force with which they travel
* Potential difference
* Opposition to current flow
* Impedance or resistance
* Direction of travel
* Alternating current/Direct current
* Conventional electric current is described as going from positive to negatives poles
* Electron flow is the movement from negative poles to positive poles
* Quantity of electrons flowing
* Also known as current
* Force with which they travel
* Potential difference
* Opposition to current flow
* Impedance or resistance
* Direction of travel
* Alternating current/Direct current
73
New cards
4 things that influence how electrons flow
1. Direction of travel
2. Current
3. potential difference
4. resistance
74
New cards
Directions
* the direction of travel for electrons will either be direct current (DC) or alternating current (AC)
* DC - electrons move in one direction
* AC - electrons move in one directions then reverse that direction and move in opposite direction
* DC - electrons move in one direction
* AC - electrons move in one directions then reverse that direction and move in opposite direction
75
New cards
Current
* the quantity or # of electrons flowing
* Current is measured in amperage
* X-ray machines use milliampered units tp regulate the # of electrons available to produce x-ray photons
* Current is measured in amperage
* X-ray machines use milliampered units tp regulate the # of electrons available to produce x-ray photons
76
New cards
Current is measured in
amperage
77
New cards
Potential difference
* force or strength of electron flow
* unit of potential difference is volt
* Electrons move from highest concentration to lowest to keep balance
* this means there is excess of electrons at one end and a deficiency at the other end
* unit of potential difference is volt
* Electrons move from highest concentration to lowest to keep balance
* this means there is excess of electrons at one end and a deficiency at the other end
78
New cards
unit of potential difference is
volt
79
New cards
Resistance
* the amount of opposition to the current within the circuit
* Unit of resistance is ohm
* resistance and conductors go hand in hand
* 3 important things when choosing a conductor
1. length
2. diameter
3. temperature
* Unit of resistance is ohm
* resistance and conductors go hand in hand
* 3 important things when choosing a conductor
1. length
2. diameter
3. temperature
80
New cards
The unit of resistance is
ohm
81
New cards
Properties of conductors
* temperature - heat creates resistance meaning if the conductor gets hot, there will be a lot of resistance, if the conductor stays cool there will be less resistance
* For x-ray purposes, we want less resistance, max # of electrons to flow to create as many x-ray photons we need to make an image
* The length of the conductor is directly proportional to the resistance
* If the length of conductor doubles, the resistance doubles, same for cutting in half
* The diameter of a conductor is inversely related to resistance
* If diameter doubles, resistance is cut in half
* For x-ray purposes, we want less resistance, max # of electrons to flow to create as many x-ray photons we need to make an image
* The length of the conductor is directly proportional to the resistance
* If the length of conductor doubles, the resistance doubles, same for cutting in half
* The diameter of a conductor is inversely related to resistance
* If diameter doubles, resistance is cut in half
82
New cards
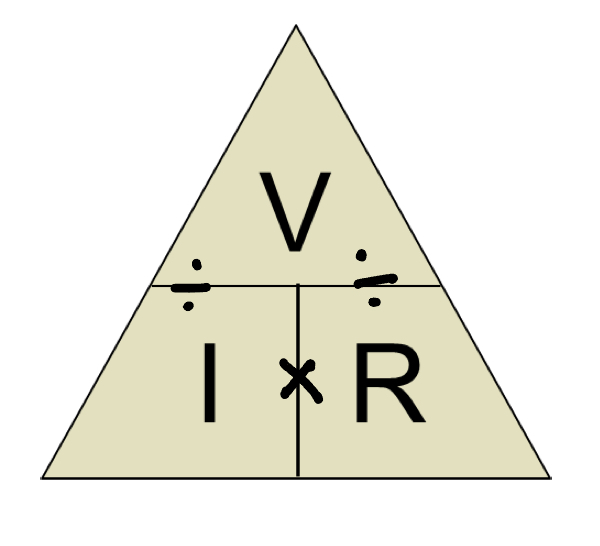
Ohms law
* George ohm discovered the mathematical relationship between current measured in amperes, potential difference measured in voltage, and resistance measured in ohms
* V= I X R
* V= potential difference (volts)
* I= current (amperes)
* R= resistance (ohms)
* V= I X R
* V= potential difference (volts)
* I= current (amperes)
* R= resistance (ohms)
83
New cards
Caluclating power
* P=IV
* P= power (watts)
* I= current (amperes)
* V= potential difference (volts)
* Math reminders
* Multiply mA by .001 because 1 mA= .001 ampere
* Multiply kV by 1000 because 1 kV= 1000 volts
* Divide watts by 1000 to reduce to kV
* P= power (watts)
* I= current (amperes)
* V= potential difference (volts)
* Math reminders
* Multiply mA by .001 because 1 mA= .001 ampere
* Multiply kV by 1000 because 1 kV= 1000 volts
* Divide watts by 1000 to reduce to kV
84
New cards
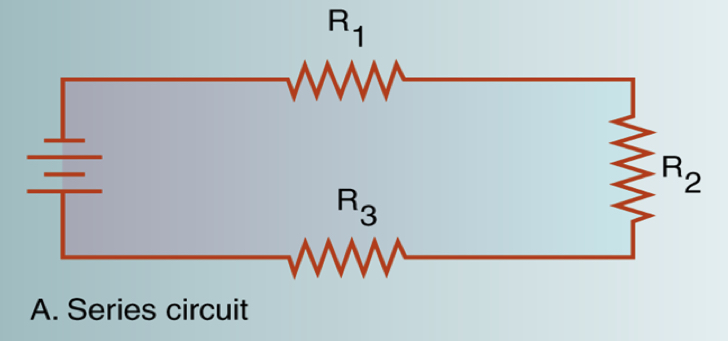
Series circuit
* A series circuit sends electrons through various resistance devices by linking them one after another in a series
* All their components connected end to end
* If one fails, the entire circuit fails
* Provides constant current
* All their components connected end to end
* If one fails, the entire circuit fails
* Provides constant current
85
New cards
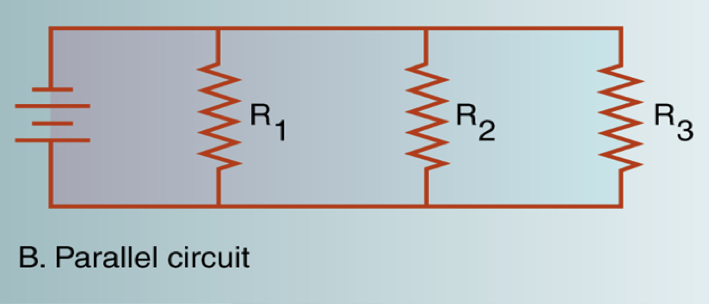
Parallel circuits
* a parallel circuit gives each component a individual branch
* each component is connected to power source
* failure in one does not disturb the entire circuit
* each component is connected to power source
* failure in one does not disturb the entire circuit
86
New cards
Total current
* Series circuit
* Same in each element, each element same as total circuit
* It=I1=I2=I3
* Parallel circuit
* Sum of all elements equals total circuit
* It=I1+I2+I3
* Same in each element, each element same as total circuit
* It=I1=I2=I3
* Parallel circuit
* Sum of all elements equals total circuit
* It=I1+I2+I3
87
New cards
Total voltage
* series circuit
* sum of all elements equals total circuits
* Vt=V1+V2+V3
* parallel circuit
* same in each element, each element same as total circuit
* Vt=V1=V2=V3
* sum of all elements equals total circuits
* Vt=V1+V2+V3
* parallel circuit
* same in each element, each element same as total circuit
* Vt=V1=V2=V3
88
New cards
Total resistance
* series circuit
* Sum of all elements equals total circuit
* Rt=R1+R2+R3
* parallel circuit
* Sum of reciprocal of each element is inversely proportional to the total
* 1/Rt= 1/R1 + 1/R2 + 1/R3
* Sum of all elements equals total circuit
* Rt=R1+R2+R3
* parallel circuit
* Sum of reciprocal of each element is inversely proportional to the total
* 1/Rt= 1/R1 + 1/R2 + 1/R3
89
New cards
magnetism (moving of atoms)
* magnetism (moving of atoms) - when a charged particle is in motion a magnetic force field perpendicular to that motion will be created
* Electromagnetism is the study of electricity and magnetism
* Magnets have moments and they are classified as orbital or spin
* Orbital magnetic moment - atoms move perpendicularly
* Spin magnetic moment - atoms spin on their axis
* Its a fundamental force and results from the motion of a charged atomic particle
* Electromagnetism is the study of electricity and magnetism
* Magnets have moments and they are classified as orbital or spin
* Orbital magnetic moment - atoms move perpendicularly
* Spin magnetic moment - atoms spin on their axis
* Its a fundamental force and results from the motion of a charged atomic particle
90
New cards
The magnetic field
* Magnetic dipoles also referred to as magnetic domain are groups of atoms that have their magnetic moments all in the same direction
* Lines of force (aka lines of flux or magnetic fiend) - created when this happens
* The more lines of force a magnet has, the stronger it is
* Magnets has North and south poles and lines of force always flow from south to north within magnet and north to south outside the magnet (they will never intersect)
* Lines of force (aka lines of flux or magnetic fiend) - created when this happens
* The more lines of force a magnet has, the stronger it is
* Magnets has North and south poles and lines of force always flow from south to north within magnet and north to south outside the magnet (they will never intersect)
91
New cards
Classifications of magnets
* Natural
* Lodestone - natural magnet that are created when iron oxide remains in the earths magnetic fields for ages and the magnetic dipoles orient themselves in same direction
* Artificial permanent
* Alnico - Manufactured from still alloy and subjected to strong commercial magnets while its still hot. This allows the magnetic dipoles to orient themselves and when the materials cool, the magnetism stays relatively permanent
* Electromagnets
* Temporary due to moving electric current
* can make a simple electromagnetic from a battery, nail and copper wire
* Lodestone - natural magnet that are created when iron oxide remains in the earths magnetic fields for ages and the magnetic dipoles orient themselves in same direction
* Artificial permanent
* Alnico - Manufactured from still alloy and subjected to strong commercial magnets while its still hot. This allows the magnetic dipoles to orient themselves and when the materials cool, the magnetism stays relatively permanent
* Electromagnets
* Temporary due to moving electric current
* can make a simple electromagnetic from a battery, nail and copper wire
92
New cards
Three laws of magnetism
* repulsion-attraction
* inverse square law
* magnetic poles
* inverse square law
* magnetic poles
93
New cards
Repulsion-attraction
* like poles repel; unlike poles attract
* like poles repel, opposites attract
* Like lines of force repel, unliked lines of force attract
* like poles repel, opposites attract
* Like lines of force repel, unliked lines of force attract
94
New cards
Inverse square law
* the force between 2 magnetic fields is directly proportional to the product of their magnitudes and inversely proportional to the square of the distance between them
* In other words… the closer an object is to a magnetic field the more influence that magnetic field will have on the object and as objects get further away from the magnetic field. The influence decreases because of the greater area between the magnetic field and the object
* In other words… the closer an object is to a magnetic field the more influence that magnetic field will have on the object and as objects get further away from the magnetic field. The influence decreases because of the greater area between the magnetic field and the object
95
New cards
Magnetic poles
* you cannot have a magnet w/ only one pole
* if you continue to cut magnets in half, each piece will have both a north and south poles
* if you continue to cut magnets in half, each piece will have both a north and south poles
96
New cards
Magnetic classification of materials (how certain materials react to magnets)
* Permeable - if a material easily becomes magnetized
* The ease with which material can be magnetized
* Retentivity
* The ability of a material to stay magnetized
* Four classifications - classified by permeability
* Ferromagnetic - highly permeable and attracted to magnetic fields
> EX: iron, cobalt, and nickel
* Paramagnetic - low permeability and weak attraction to magnetic fields
> EX: aluminum
* Diamagnetic - repelled by magnetic fields and weakly repelled by ll magnetic fields
> EX: beryllium and lead
* Nonmagnetic - not affected by magnetic fields and cannot be magnetized
> most materials fall under nonmagnetic category
>
> EX: wood, glass, rubber and plastic
* The ease with which material can be magnetized
* Retentivity
* The ability of a material to stay magnetized
* Four classifications - classified by permeability
* Ferromagnetic - highly permeable and attracted to magnetic fields
> EX: iron, cobalt, and nickel
* Paramagnetic - low permeability and weak attraction to magnetic fields
> EX: aluminum
* Diamagnetic - repelled by magnetic fields and weakly repelled by ll magnetic fields
> EX: beryllium and lead
* Nonmagnetic - not affected by magnetic fields and cannot be magnetized
> most materials fall under nonmagnetic category
>
> EX: wood, glass, rubber and plastic
97
New cards
The study of magnetism
* Hans Orsted
* His experiment led to the discovery that any moving charge produces a magnetic field and that magnetic field is always perpendicular to the direction of the moving charge
* 1820 - orsted discovered the relationship between moving electric charge and magnetism
* His experiment led to the discovery that any moving charge produces a magnetic field and that magnetic field is always perpendicular to the direction of the moving charge
* 1820 - orsted discovered the relationship between moving electric charge and magnetism
98
New cards
Flemings hand rules
* four categories - these rules are important to knowing how a generator and motor work
1. hand thumb rules along conductor
2. Hand thumb rules for solenoid and electromagnet poles
3. Right hand generator rule
4. Left hand motor rule
1. hand thumb rules along conductor
2. Hand thumb rules for solenoid and electromagnet poles
3. Right hand generator rule
4. Left hand motor rule
99
New cards
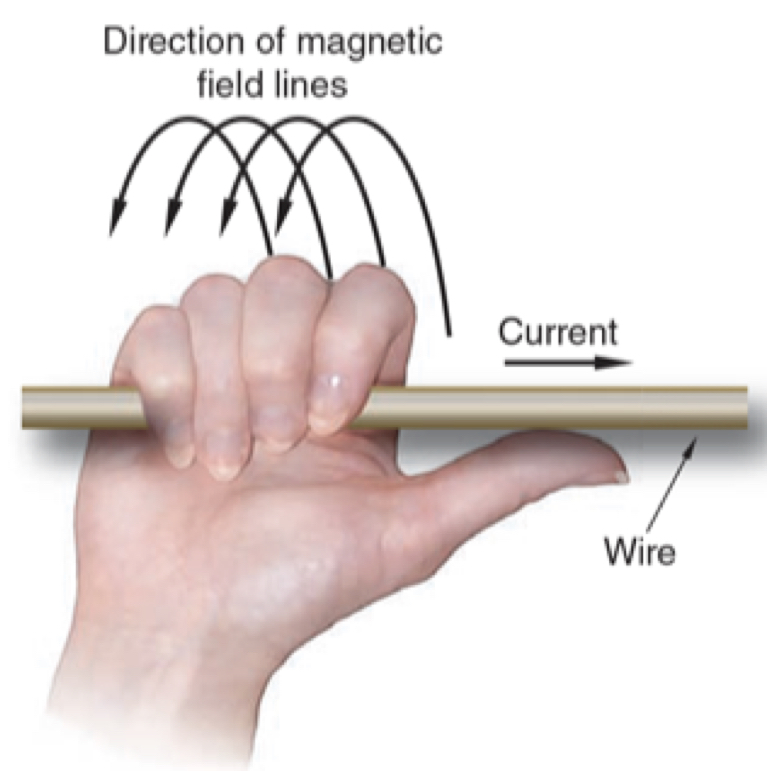
Right hand thumb rule (for a straight conductor)
* Applies to conventional current flow
* Conventional electrical current flows positive to negative
* Conventional electrical current flows positive to negative
100
New cards
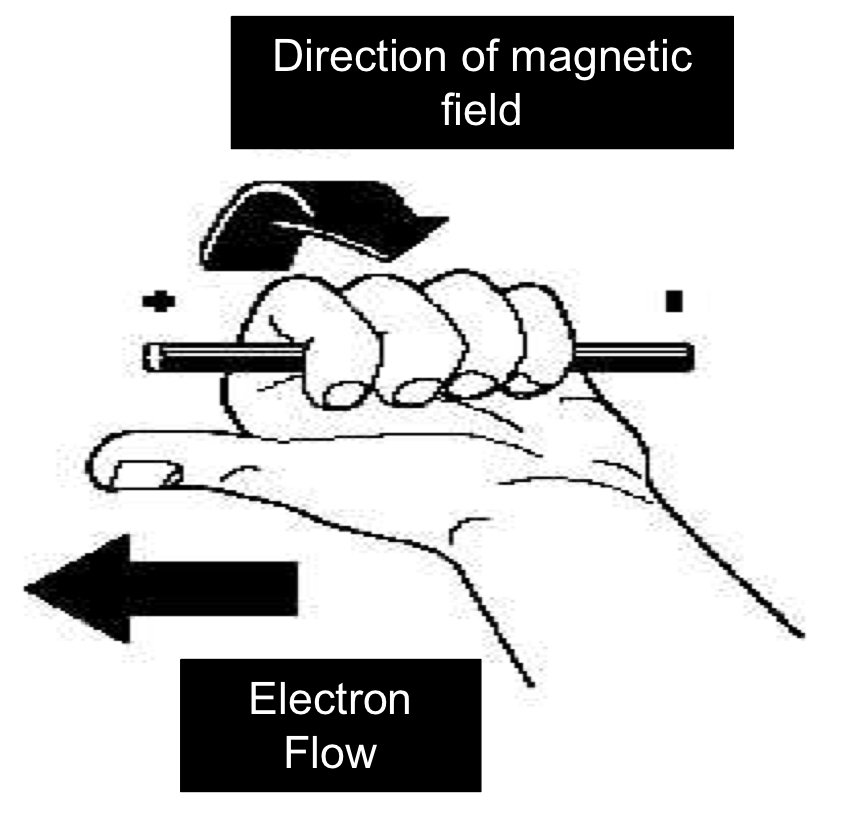
Left hand thumb rule
* applies to electron current flow
* electron current flow goes from negative to positive
* electron current flow goes from negative to positive