topic 1 - energy
1/51
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
describe all the changes in energy involved when an object is projected upwards
the object’s store is initially kinetic store as it is moving upwards
the object’s energy is slowly transferred to the gravitational potential store
as it is slowing down and gaining a higher altitude
all the energy will be transferred to the gravitational potential store when the object has hit it’s altitude peak
describe all the changes in energy involved when a moving object hits an obstacle
the object’s store is initially kinetic store as it is moving
when the object collides with the obstacle, energy is immediately converted to
the kinetic store of the obstacle
and the thermal stores of the object and obstacle
some energy remains in the object’s kinetic store as it moves away from the obstacle immediately after collision
describe all the changes in energy involved when an object is accelerated by a constant force
work is done by a force on the object
the work done converts to the kinetic store of the object
describe all the changes in energy involved when a vehicle is slowing down
the vehicle’s energy is initially in the kinetic store
the brakes do work slowing the vehicle down
where energy is dissipated into the surroundings
by heat and sound
describe all the changes in energy involved when a water is brought to a boil in an electric kettle
energy transfers from the electrical store of the mains supply
to the thermal store of the water
describe all the changes in energy involved in heating
heating an object transfers energy to the object’s internal thermal store
state the definition of an internal store of energy
the sum of the energy stored in the kinetic and chemical potential of an object’s particles
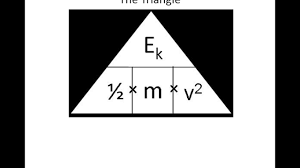
state the equation determining the amount of energy associated with a moving object
kinetic energy (J) = ½ x mass (kg) x velocity² (m/s)
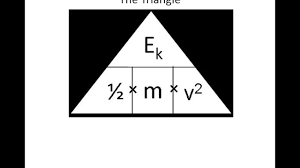
state the symbol equation determining the amount of energy associated with a moving object
E (J) = ½ x m (kg) x v² (m/s)
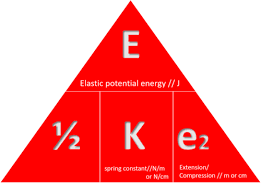
state the equation determining the amount of energy associated with a stretched spring
elastic potential energy (J) = ½ x spring constant (N/m) x extension² (m)
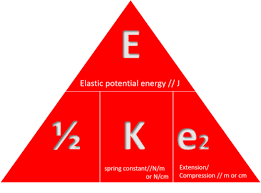
state the symbol equation determining the amount of energy associated with a stretched spring
E (J) = ½ x k (N/m) x e² (m)
state the equation determining amount of energy associated with an object raised above ground level
gravitational potential energy (J) = mass (kg) x gravitational field strength (N/kg) x height (m)
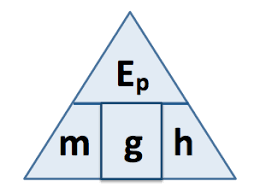
state the symbol equation determining amount of energy associated with an object raised above ground level
E (J) = m (kg) x g (N/kg) x h (m)
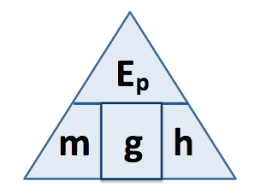
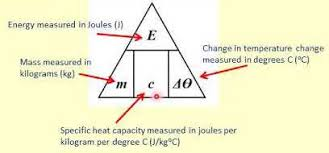
state the equation determining the amount of energy stored in or released from a system during a temperature change
change in thermal energy (J) = mass (kg) x specific heat capacity (J/kg°C) x temperature change (°C)
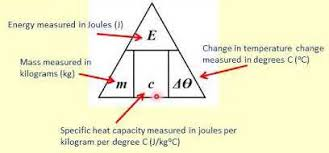
state the symbol equation determining the amount of energy stored in or released from a system during a temperature change
E (J) = m (kg) x c (J/kg°C) x ΔT (°C)
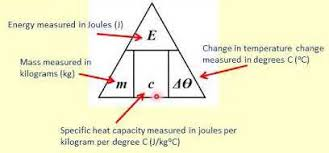
state the independent variable in the investigation of specific heat capacity in different materials practical
time
state the dependent variable in the investigation of specific heat capacity in different materials practical
temperature
state the control variables in the investigation of specific heat capacity in different materials practical
material of the block
current supplied
potential difference supplied
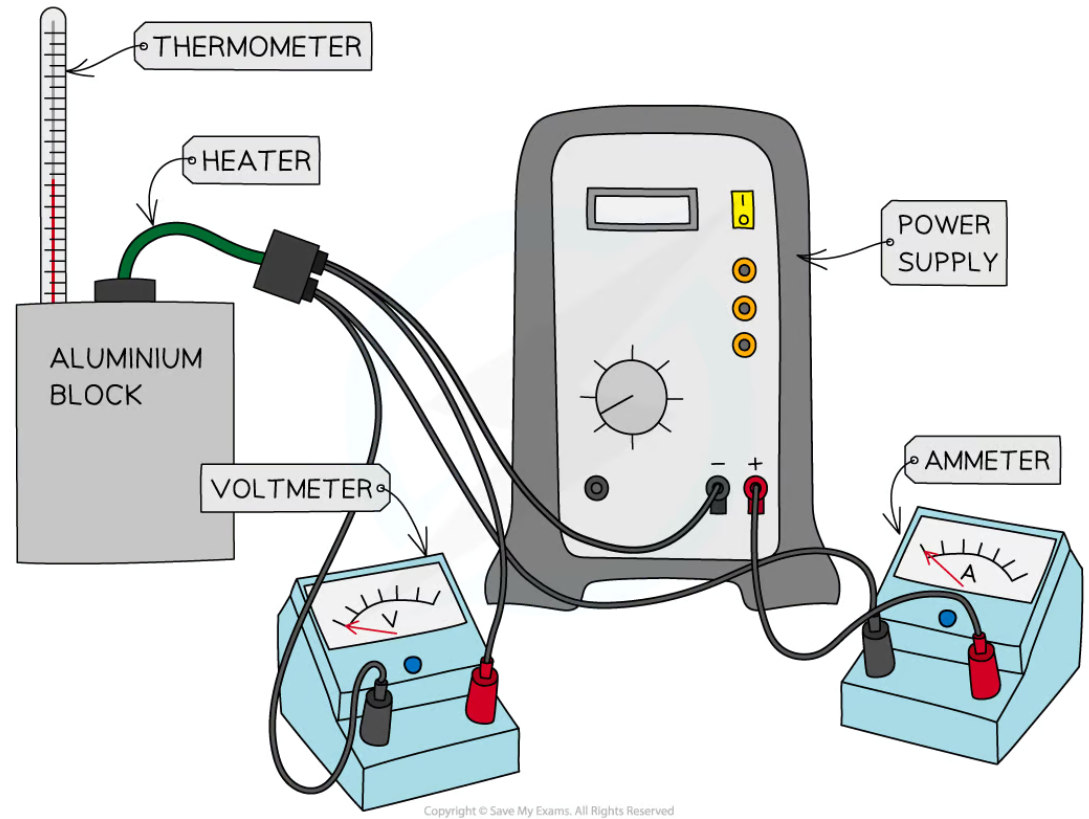
specific heat capacity in different materials practical (method)
apparatus = thermometer and heater connected to an aluminium block, power supply, ammeter in series, voltmeter in parallel
measure initial temperature of aluminium block using thermometer
turn on power supply, start the stopwatch
take periodic measurements of current and voltage from ammeter and voltmeter until stopwatch reaches 10 minutes
calculate voltage and current average at end of experiment
switch off power supply, stop stopwatch, leave the apparatus to cool for a minute
record final temperature of aluminium
specific heat capacity in different materials practical (analysis of results)
change in thermal energy (J) = mass (kg) x specific heat capacity (J/kg°C) x temperature change (°C)
specific heat capacity in different materials practical (evaluation of experiment)
zero error - ensure voltmeter and ammeter are at zero before the experiment
random error - not all energy transferred from the heater will be transferred to the block as some thermal energy is dissipated into the surroundings
state the definition of power
power is the rate at which energy is transferred/work is done
state the equation linking power, energy and time
power (W) = energy (J) / time (s)
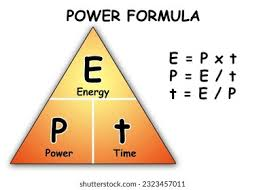
state the symbol equation linking power, energy and time
P (W) = E (J) / t (s)
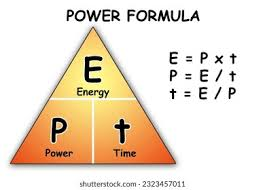
state the equation linking power, work done and time
power (W) = work done (J) / time (s)
state the symbol equation linking power, work done and time
P (W) = E (J) / t (s)
state how to convert an energy transfer in Joules to Watts
1 Joule per second = 1 Watt
state the properties of energy
it can be transferred usefully
it can be stored
it can be dissipated
it cannot be created or destroyed
state what occurs when there are energy transfers in closed systems
there is no net change in the total energy
state how to reduce unwanted energy transfers using lubrication (bike example)
friction causes energy dissipation through thermal energy lost to the surroundings
energy is transferred from the kinetic energy store of the bike to the thermal energy store of the gears and chain
friction makes them become hot and transfers energy by heating to the thermal energy store of the surrounding air
lubricating the gears and chain reduces friction
which reduces the loss of thermal energy
state how to reduce unwanted energy transfers using thermal insulation
insulation stops thermal energy from dissipating into the surrounding air
meaning less energy will be needed to replace the wasted energy
this is useful in domestic heating and boiling a kettle
state the relationship between thermal conductivity of a material and the rate of energy transfer by conduction
the higher the thermal conductivity of a material
the higher the rate of energy transfer by conduction across the material
so they are directly proportional
state how the rate of cooling of a building is affected by the thickness of the walls
thicker walls transfer thermal energy by conduction slower than thinner walls
because the added material in thick walls decreases the thermal conductivity of the wall
slowing the rate of thermal energy transfer through the wall
state how the rate of cooling of a building is affected by the thermal conductivity of the walls
the lower the thermal conductivity of the wall
the slower its rate of cooling
as it takes longer for the thermal energy to dissipate into the surrounding air
state the independent variable in the investigation of thermal insulation in different materials practical
type of material
state the dependent variable in the investigation of thermal insulation in different materials practical
temperature
state the control variables in the investigation of thermal insulation in different materials practical
volume of water
initial temperature of water
thickness of each material
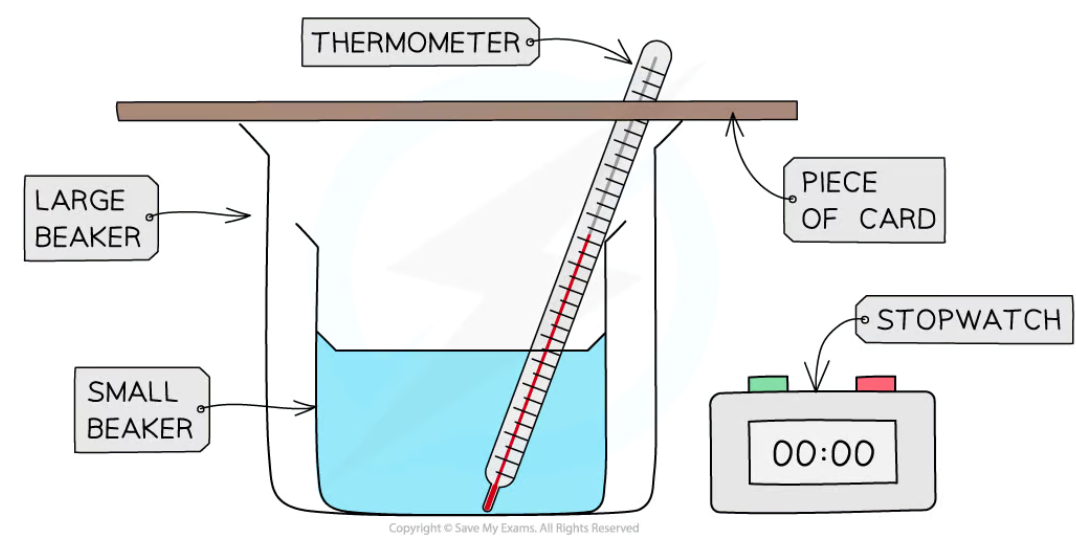
investigation of thermal insulation in different materials (method)
set up the apparatus by placing a small beaker with a thermometer in it in a big beaker
fill the small beaker with boiling water from a kettle
place a piece of cardboard over the beakers as a lid, with a small hole in it for the thermometer
record the initial temperature of the water and start the stopwatch
record the temperature of the water every 2 minutes for 20 minutes or until the water reaches room temperature
repeat the experiment using different materials as the lid and without any lid at all (the control)
investigation of thermal insulation in different materials (results)
create a graph for the temperature change by material
the graph should show that the temperature falls quicker at higher temperatures
and temperature loss is more gradual at lower temperatures
the curve which takes the longest for the temperature to drop is the best insulator
investigation of thermal insulation in different materials (analysis of results)
the temperature falls quicker at higher temperatures as there is a greater temperature difference between the water temperature and room temperature
this means there is a greater energy transfer by heating
investigation of thermal insulation in different materials (evaluation of experiment)
systematic error - only the top of the beaker is covered, meaning some thermal energy is dissipated into the surrounding air through conduction in the beaker walls
parallax error - read the values on the thermometer at eye level otherwise the parallax error is introduced
state how to calculate the efficiency of any energy transfer
efficiency = useful energy output (W) / total energy output (W)
state how to increases the efficiency of an intended energy transfer
lubrication - reduces friction which reduces the dissipation of thermal energy into the surrounding air
reducing current - reduces the resistance of a circuit, reducing unwanted heat transfer to wire in the circuit
streamlining objects - reduces air resistance, which reduces unwanted energy transfer by heating into the surrounding air
insulation - reduces thermal energy dissipation into surrounding air
state the main energy resources available for use on Earth
fossil fuels
nuclear fuel
bio-fuel
wind
hydro-electricity
geothermal
tidal
solar
state the definition of a renewable energy resource
a resource that is being or can be replenished as it is used
state the uses of energy resources
transportation - fossil fuels and solar
electricity generation - all energy resources
heating - fossil fuels, geothermal, solar, wind
state which energy resources available on earth are renewable
tidal
solar
geothermal
hydro-electricity
bio-fuel
state which energy resources available on earth are non-renewable
fossil fuels
nuclear fuel
explain why some energy resources available on Earth are more reliable than others
finite resources - fossil fuels and nuclear fuel are finite resources, meaning the supply of these energy resources are limited
non-renewable resources - fossil fuels and nuclear fuels are non-renewable resources, meaning they cannot be recycled or reused for the same purpose
availability of resources - fossils fuels, solar and wind are readily available resources, meaning currently there is a larger supply of these resources than others
describe the environmental impact arising from the use of fossil fuels
during combustion, fossil fuels produce carbon dioxide, which enhances the greenhouse effect
damage is caused to natural land during the mining process for fossil fuel
during combustion, sulphur dioxide gas can be produced, forming acid rain when reacting with water in the atmosphere
describe the environmental impact arising from the use of nuclear fuel
nuclear fuel creates harmful radioactive waste when used, which causes damage to the environment
explain the patterns and trends in the use of energy resources
most of the electricity generated globally is produced by fossil fuels, as they have a higher power output than some renewable energy resources and have more existing infrastructure
in some developed countries, nuclear fuel is a growing form of electricity generation as they release large amounts of energy and no carbon dioxide