Chem 101 Unit One
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
61 Terms
Physical vs Chemical Properties
- Physical: don't change a substance's chemical makeup
- Chemical: change a substance's chemical makeup
Extensive vs. Intensive Property
- Extensive: depends on quantity of substance present (ex: mass)
- Intensive: doesn't depend on quantity of substance present (ex: color, melting point)
**"Intensive = Independent"
Law of Conservation of Energy
states that energy cannot be created or destroyed, but can change from one form to another
Kinetic vs. Potential Energy
- Kinetic Energy: energy of motion
- Potential Energy: energy by position/composition (stored energy)
Energy is ______________ when bonds are formed and ____________ when bonds are broken
released, absorbed
Sublimination
solid to gas
Deposition
gas to solid
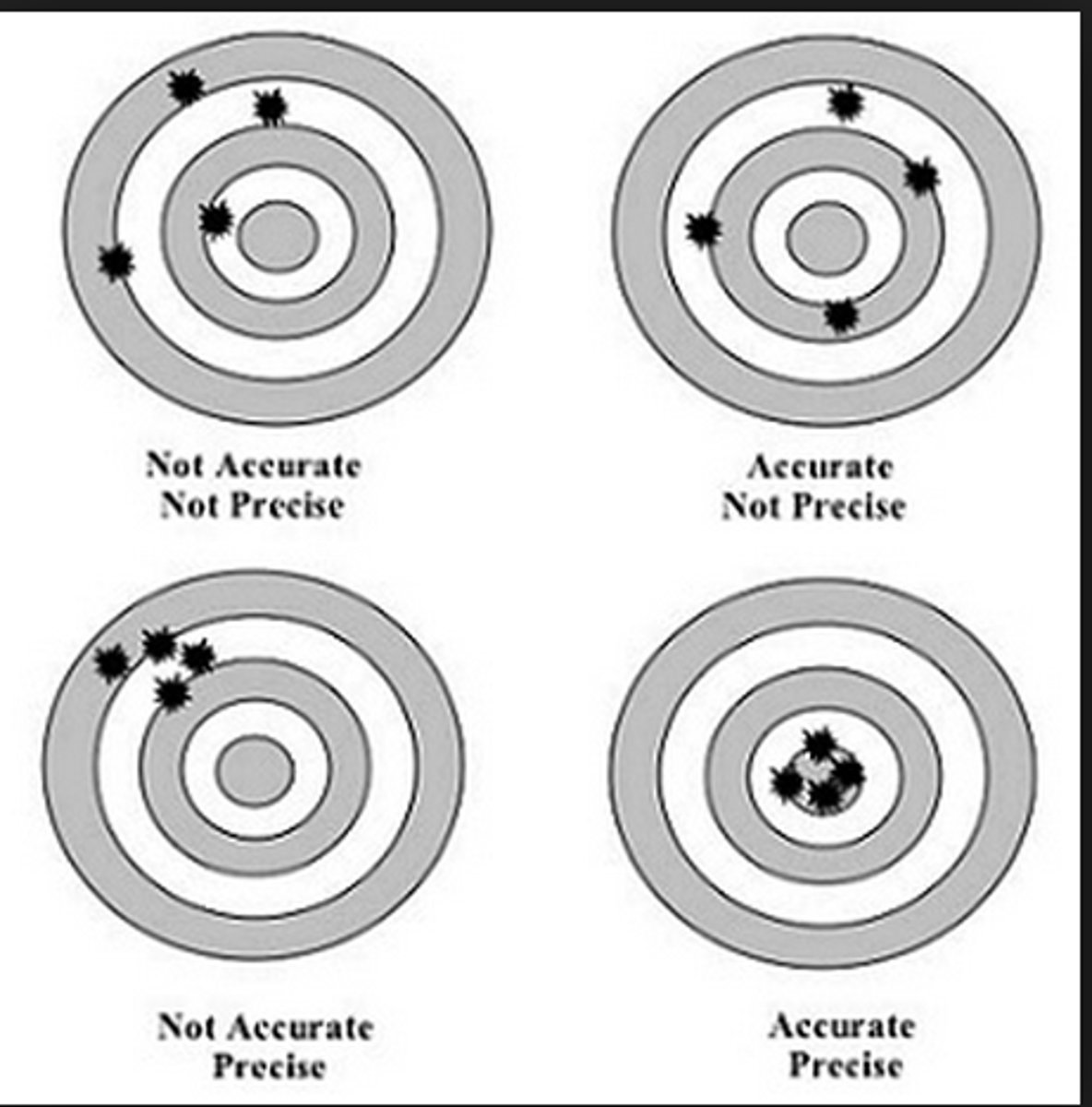
Accuracy vs. Precision
- Accuracy: how close a result is to the true value
- Precision: how repeatable the result is
Temperature
measure of the average kinetic energy of particles
Kelvin to Celsius
K = C + 273.15
Changing the temperature of something by 1 degree Celsius (is/is not) the same as changing it by one Kelvin
is (even though they're different temperature systems, a change in one produces the same change in the other; their numerical values will just be different)
More decimal places in a number means (more/less) uncertainty
less
When adding/subtracting, the final answer should have...
the same number of decimal places as the number with the LEAST number of decimal places
ex: 2.6 + 10.517 = 13.1 (one decimal place)
When multiplying/dividing, the final answer should have...
the same number of sigfigs as the number with the LEAST number of sigfigs
ex: 2.6 * 10.517 = 27 (two sigfigs)
When solving a problem and trying to account for sigfigs, know that _______ values and _________ _________ between ___________ ____________ do NOT count
exact values (have infinite sigfigs), conversion factors, metric prefixes
When solving a problem, keep track of sigfigs after every...
mathematical operation
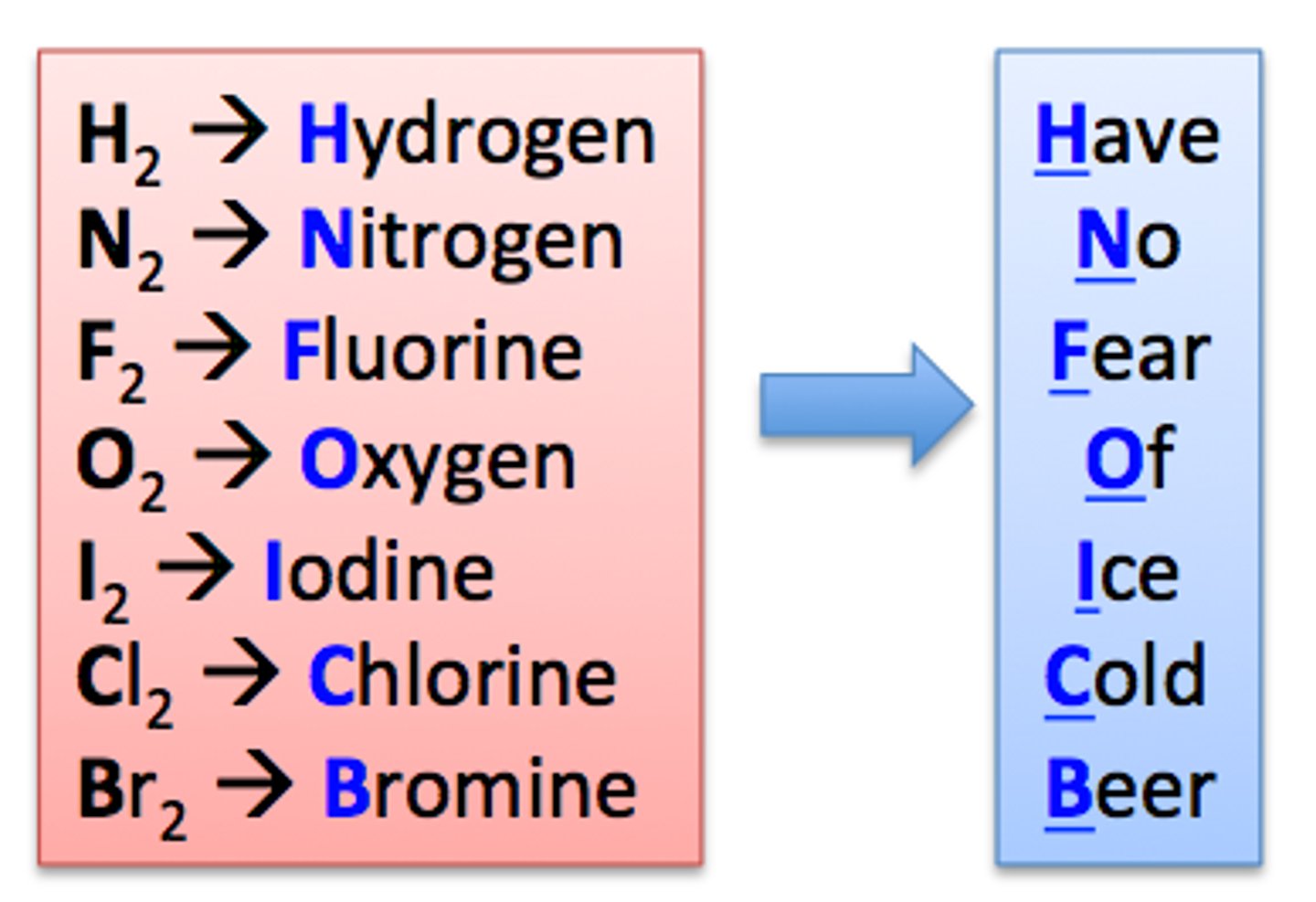
Diatomic Molecules
pure elements that exist as molecules (see image)
Cations vs. Anions
- cations = (+)
- anions = (-)
**cathode and anode are flipped
Protons vs. Neutrons vs. Electrons
- Protons: positive, in nucleus, weighs ~1.007 amu
- Neutrons: neutral, in nucleus, weight ~1.009 amu
- Electrons: negative, in electron cloud, weighs ~0 amu
Nuclide
fancy term for isotope
Thomson's Cathode Ray Tube Experiment
discovered the electron + its charge
Millikan's Oil Drop Experiment
discovered mass of electron
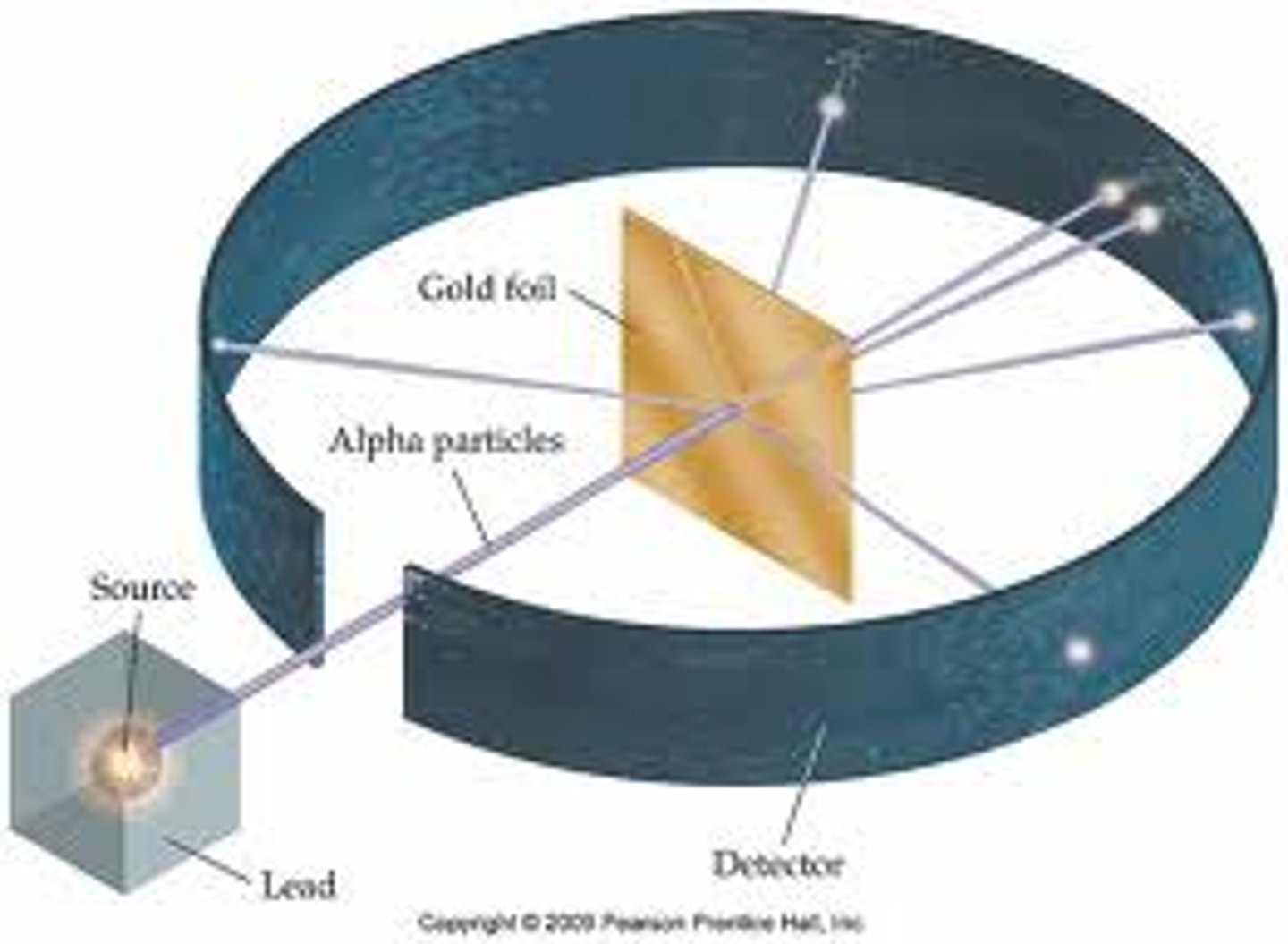
Rutherford's Gold Experiment (describe the original hypothesis, what happened during the experiment, and what they concluded from it)
- bombarded gold sheet with alpha (a) particles, which were positively charged
- thought that all a particles would go through b/c they assumed the "plum-pudding" model was correct (thought atom was a sphere of weak positive matter with electrons embedded throughout)
- some of the particles were deflected at large angles, indicating a region of dense positive charge (led to discovery of nucleus + nuclear view of atom)
When ionizing an element (changing its charge), you are changing its number of ________________.
electrons
Average Atomic Mass
weighted average of the masses of all of an element's existing isotopes
Isotope Symbol (format)
- mass number = number of protons + neutrons
- atomic number = number of protons
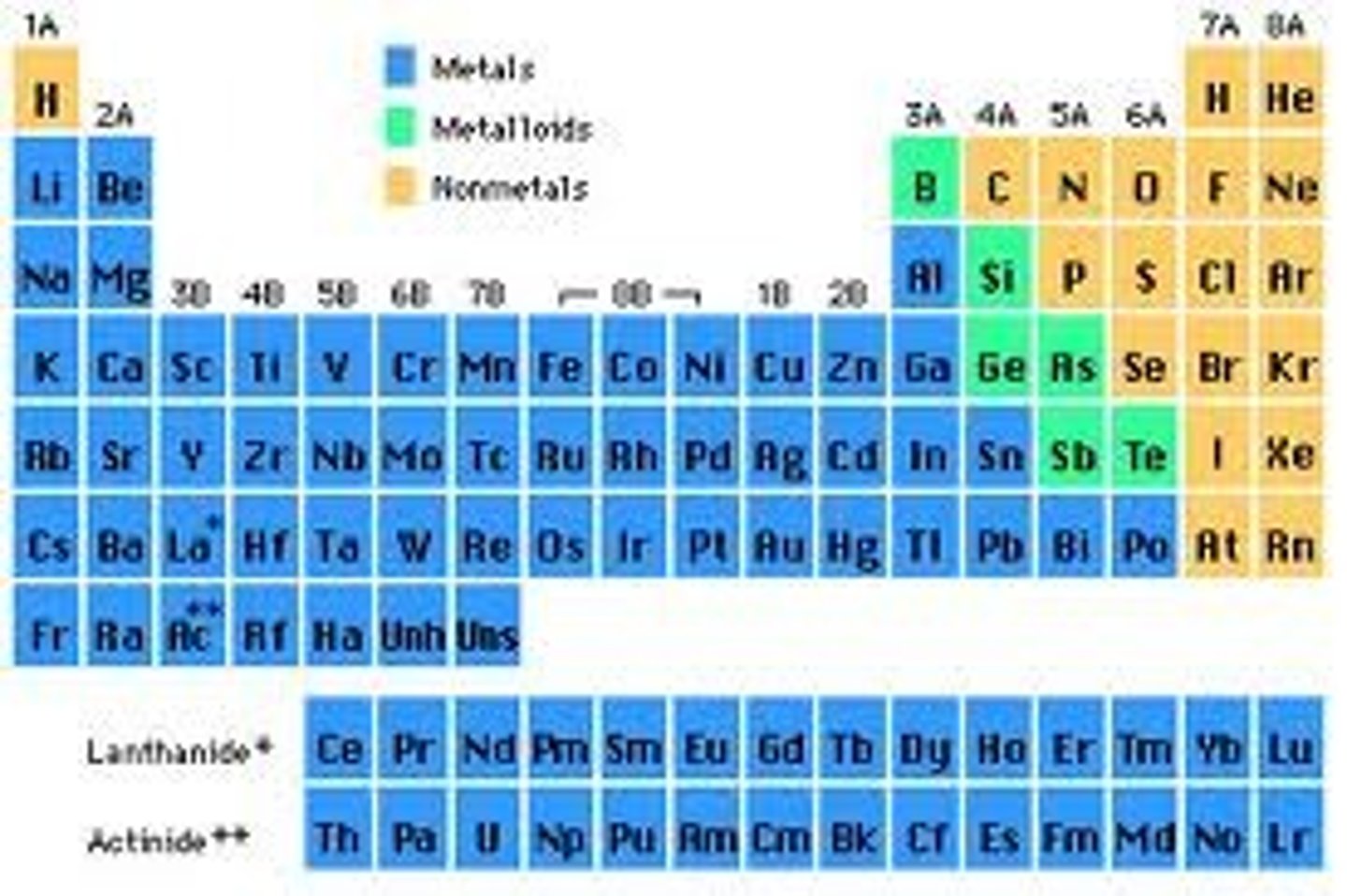
Metals (describe their properties)
- shiny and solid
- conductors of heat + electricity
- malleable (able to be hammered)
- ductile (able to be drawn out into thin wires)
**Mercury is an exception (is liquid)
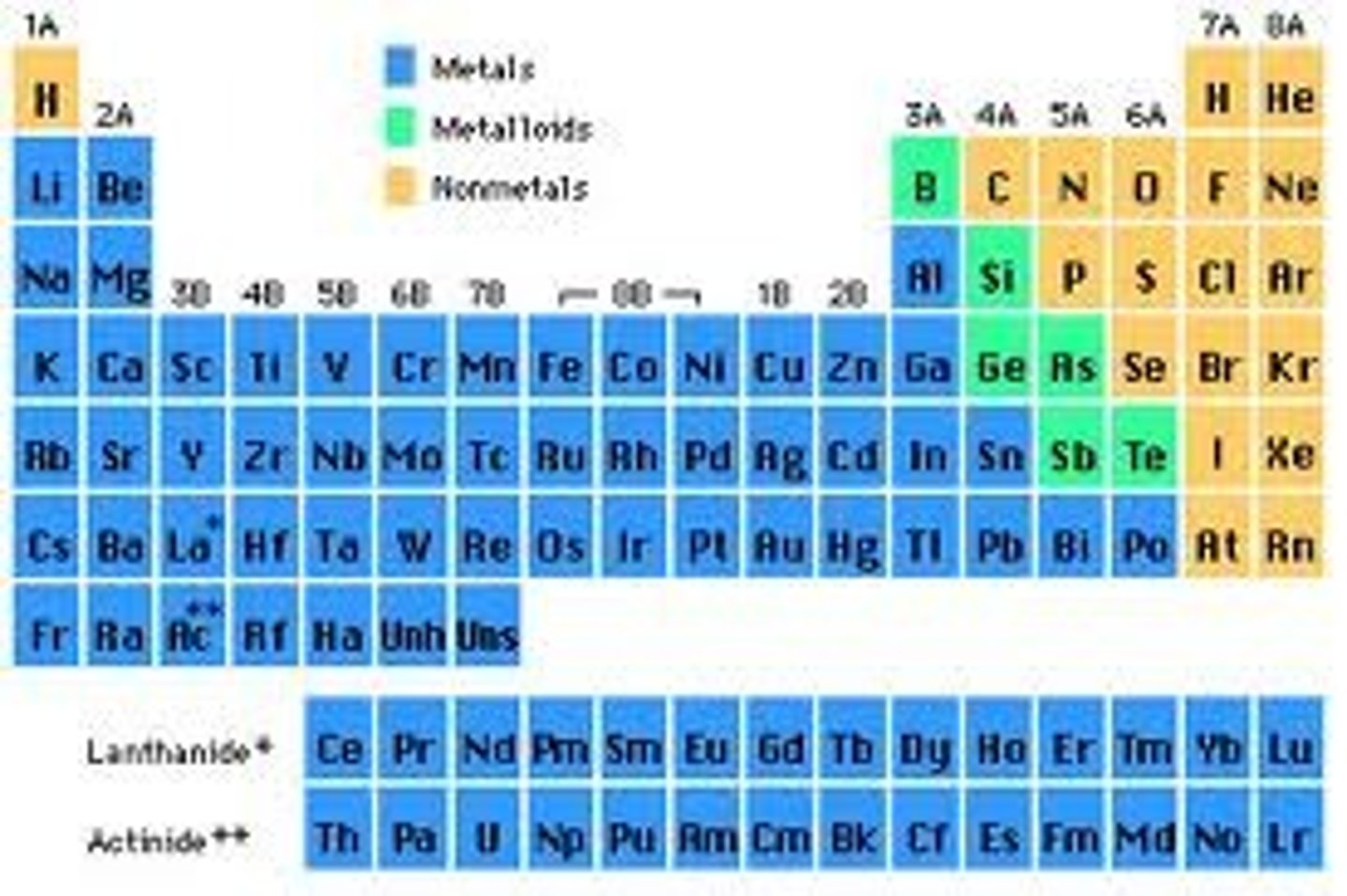
Nonmetals (describe their properties)
- solids (brittle), liquids, and gases
- non-conductors
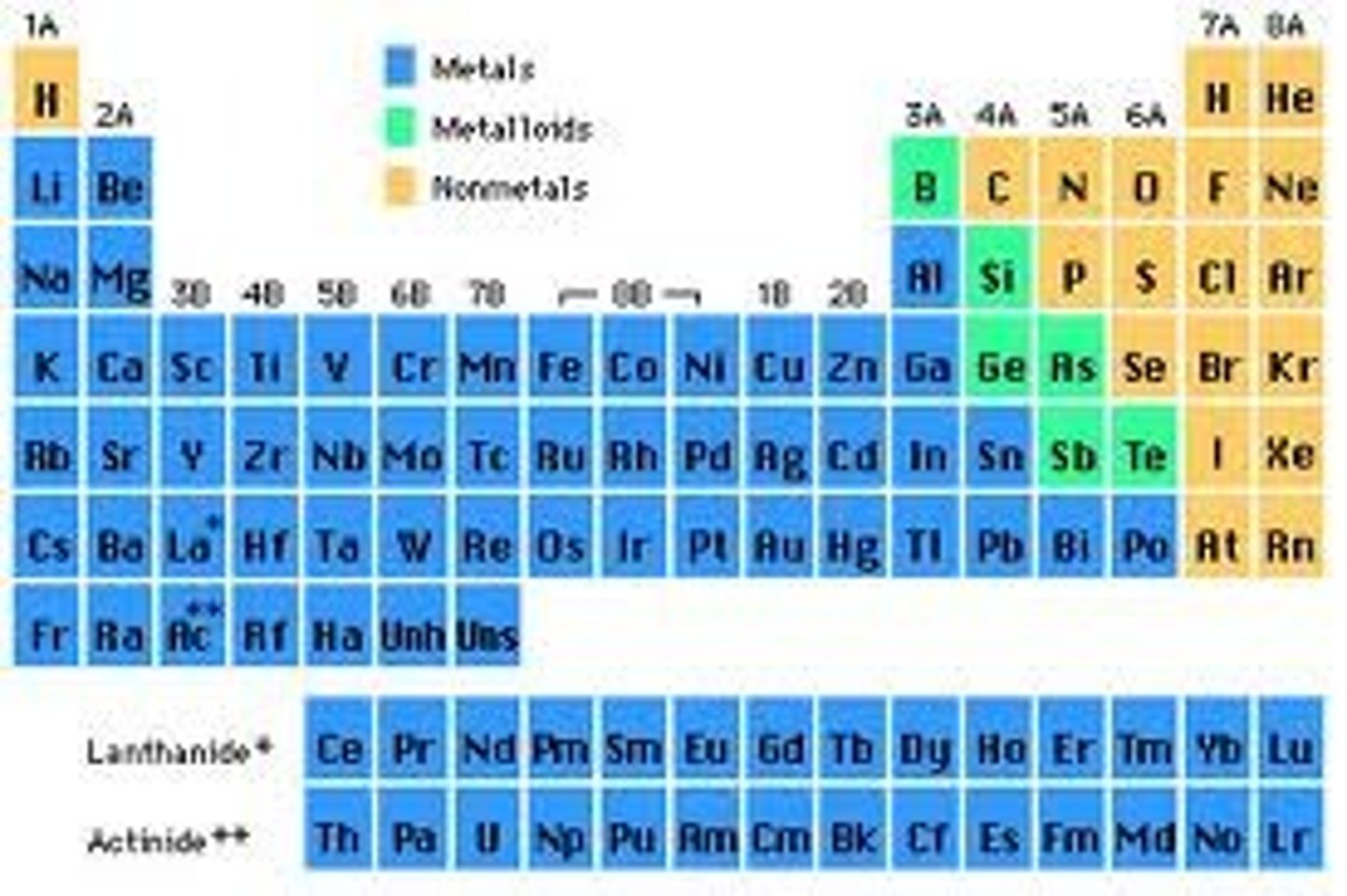
Metalloids (describe their properties
- shiny (like metals) but brittle (like nonmetals)
- semi-conductors
Mole
contains 6.022 10^23 (Avogadro's Constant*) atoms of a substance
Molecular Mass (what is it and what types of compounds DON'T have this?)
- sum of the masses of all of the atoms in a molecule (unit: u/molecule)
- ionic compounds DON'T have molecular mass b/c they're not made of molecules; instead have formula mass instead
Molar Mass (what is it and how does it compare to atomic/molecular mass?)
- mass (in grams) of one mole of an element
- it has the same NUMERICAL value as the atomic/molecular mass, but its unit is g/mol
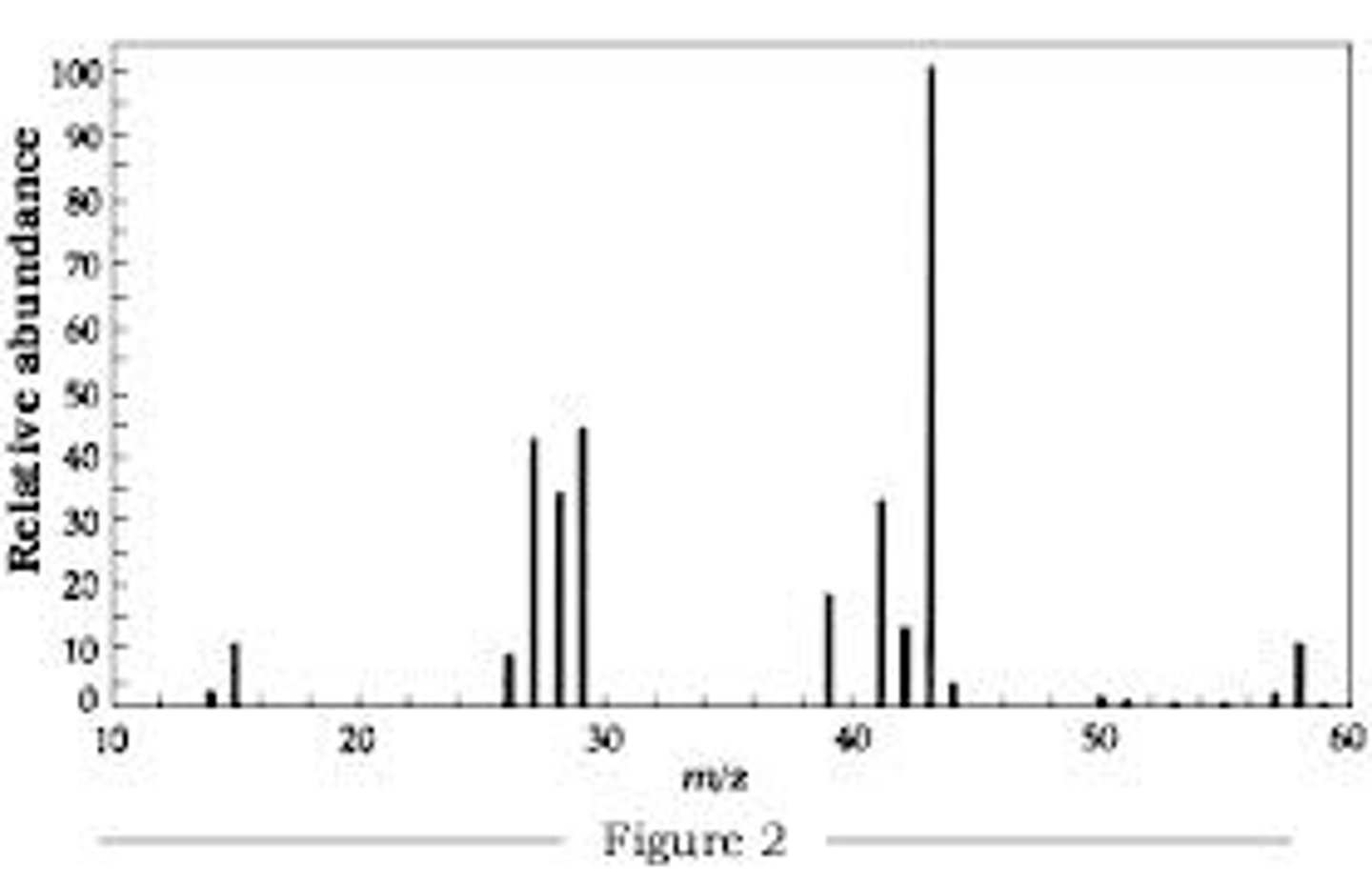
Mass Spectrum Graph
- showcases relative abundance of each isotope
- x-axis is usually m/z (with z being one) or just mass (in u)
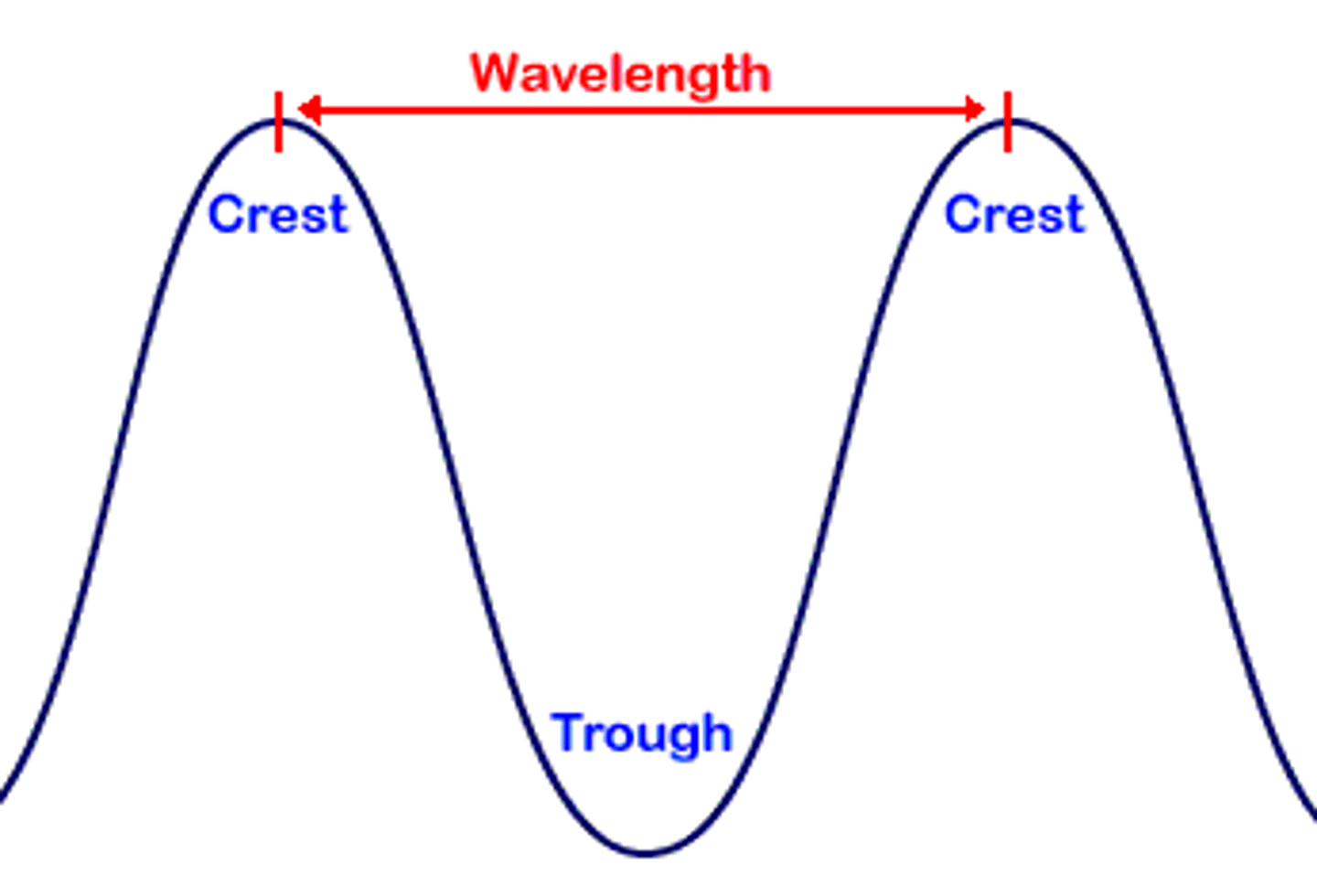
Wavelength (λ)
distance from one crest to the next
Frequency (describe its unit)
- the number of wavelengths that pass a fixed point each second
- unit = Hz (or s^-1 or 1/s)
What is the formula relating speed of light, frequency, and wavelength?
v = c/λ (any other variations of this also work!)
- v = frequency (in hz)
- c = speed of light (2.998 * 10^8 m/s)
- λ = wavelength (in meters)
Energy of a Photon (describe its equation(s), what each symbol represents, and the units that the energy is measured in)
E = hv OR E = h(c/λ)
- h = planck's constant (6.626 10^-34 Js)
- c = speed of light (2.998 * 10^8 m/s)
- v = frequency (in hz)
- λ = wavelength (in meters)
- E = energy in J/photon
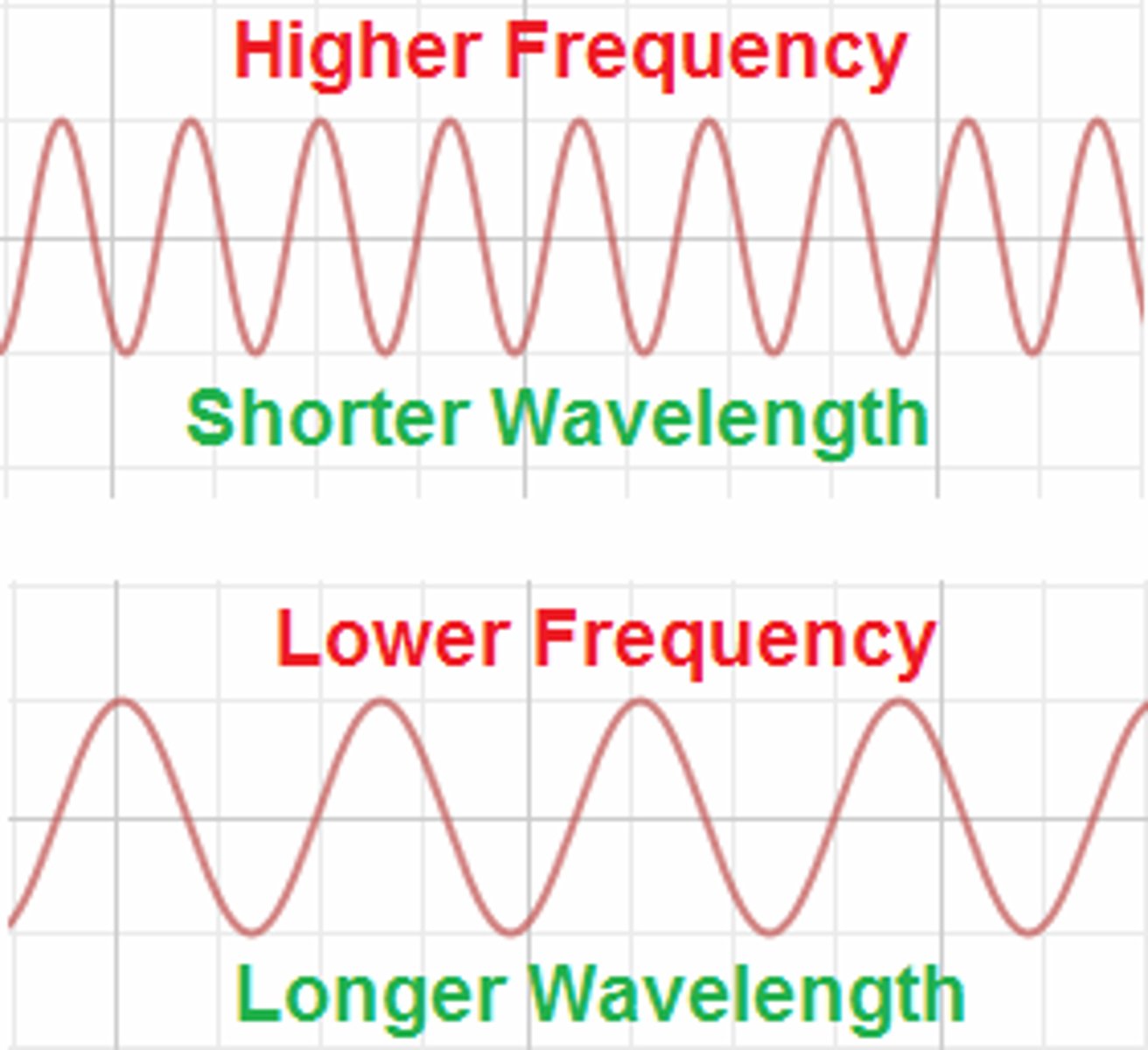
How are wavelength, frequency, and the energy of a photon related?
- Frequency and energy are directly proportional
- Frequency and wavelength are inversely proportional
- Energy and wavelength are inversely proportional
ex: as wavelength increases, frequency and energy decrease
ex: as wavelength decreases, frequency and energy increase
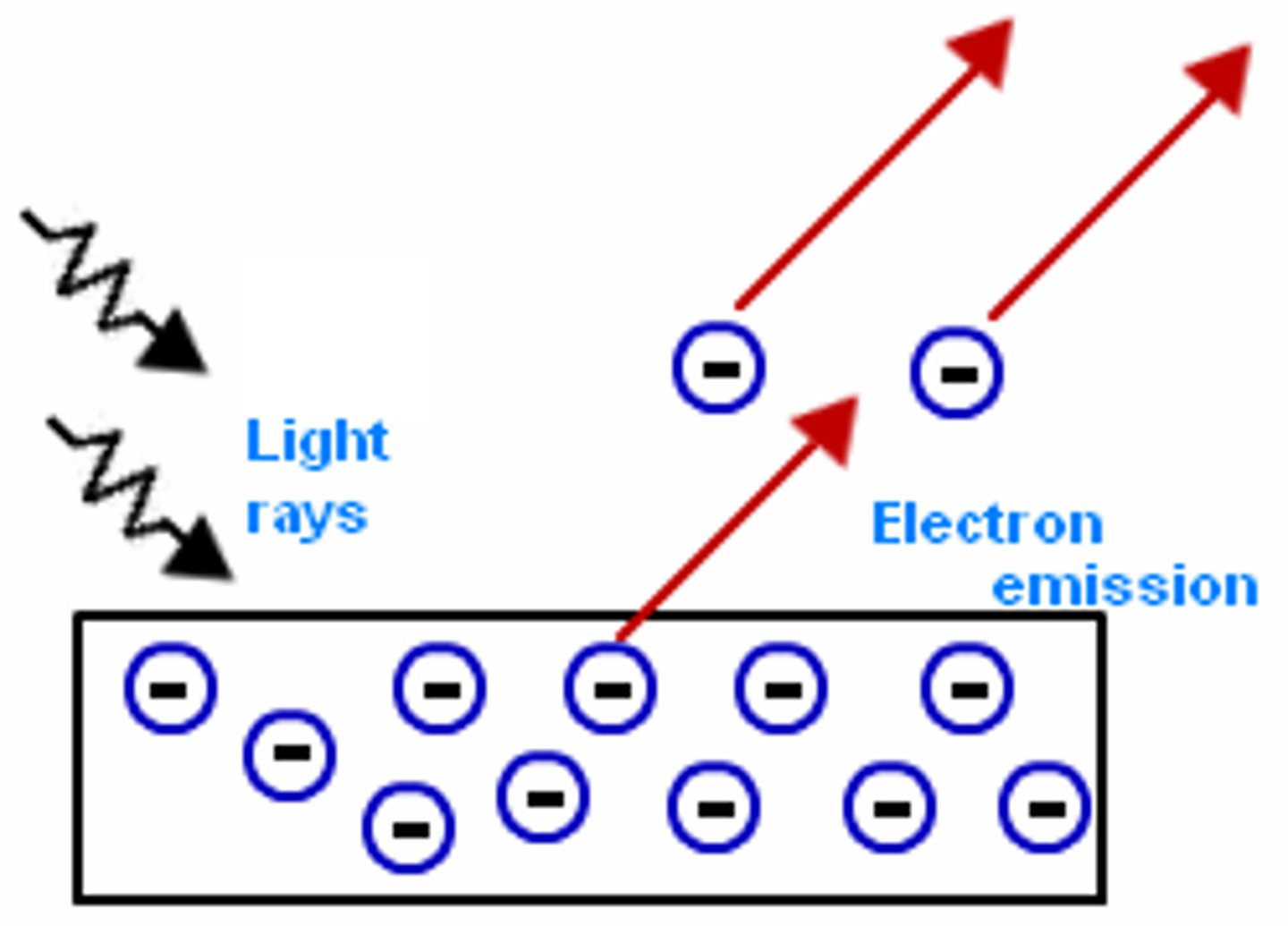
Work Function
represents the minimum amount of energy needed to dislodge an electron from the surface of a material
What happens when excess energy (energy greater than the work function) hits the surface of a material?
the excess energy provides the electron being dislodged with more kinetic energy
Photoelectric Effect (what do we call the electrons that are involved in this effect?)
- occurs when metals/semi-conducters are illuminated and electrons are emitted
- these electrons are referred to as photoelectrons
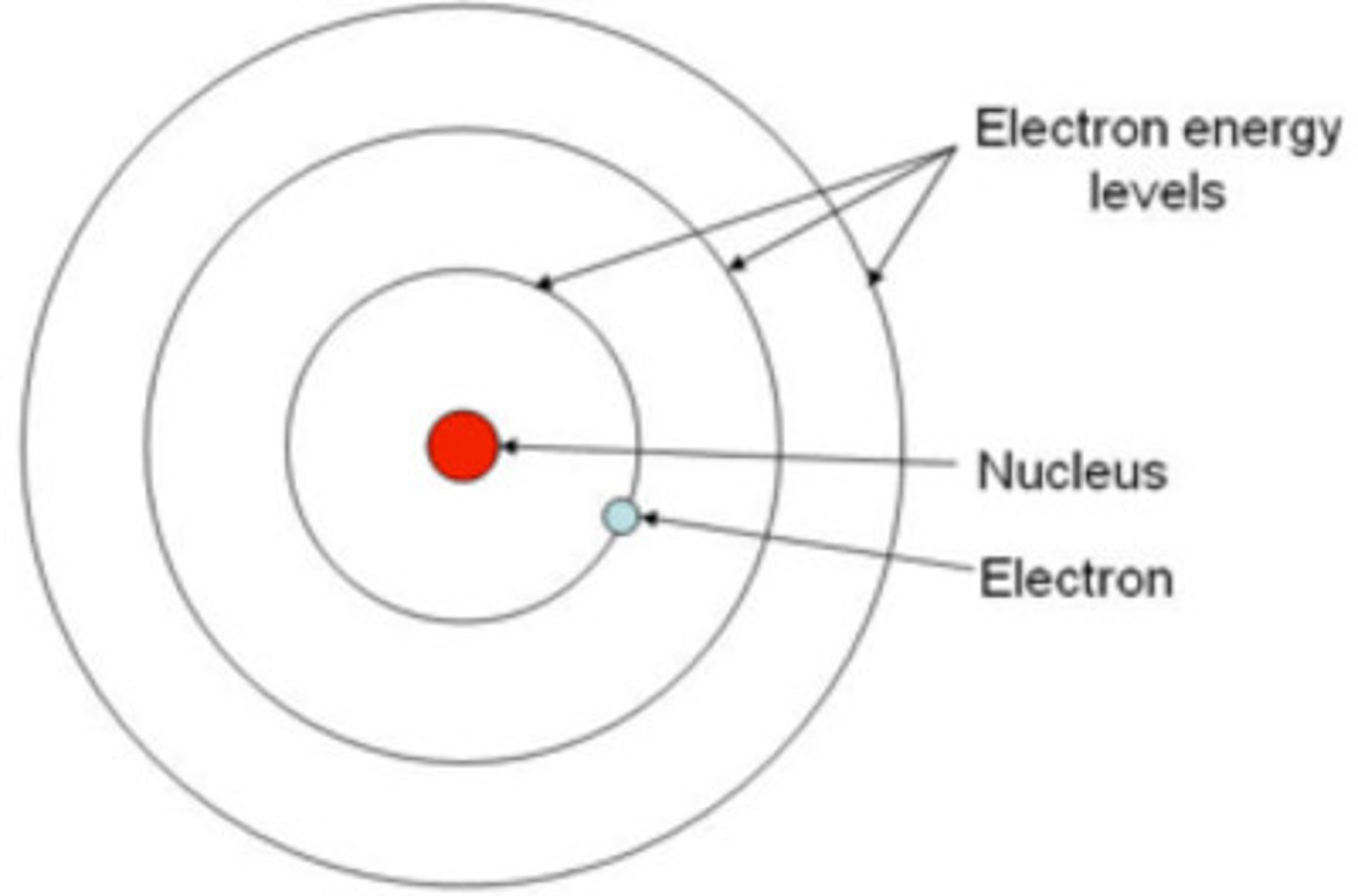
Bohr Model (how does it work + what type of atoms does it apply to?)
- planetary model that displays various energy levels that electron can be at
- only applies to hydrogen-like atoms (atoms with one electron)
In Bohr's Model, electrons ________ energy when traveling to higher levels and ________ energy (which releases _____________) when going back to their ground state
absorb, emit, photons
Ground State vs. Excited State
- ground state = lowest energy, closest to the nucleus
- excited state = higher energy, when electron is farther from nucleus (n =/= 1)
Matter Waves (describe equation)
waves associated with moving particles
- λ = wavelength (in meters)
- h = planck's constant (6.626 10^-34 Js)
- m = mass (kg)
- v = speed/velocity
**mv = momentum
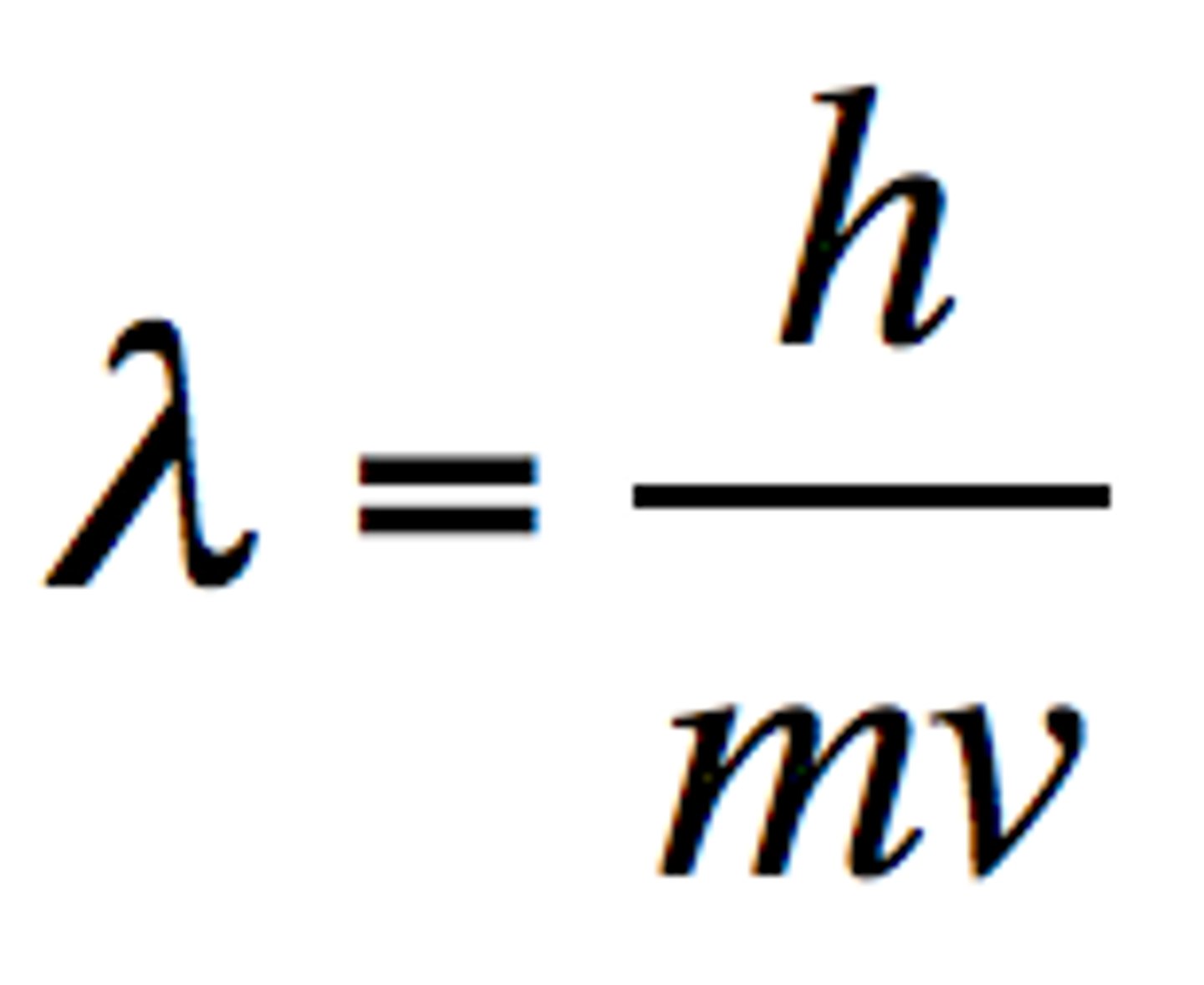
As the mass or speed of an object (its momentum) increases, its wavelength...
decreases
Standing Wave (what is it + how is it important in the structure of an atom?)
- wave that oscillates back and forth within a fixed space
- keeps electrons from being pulled into the nucleus
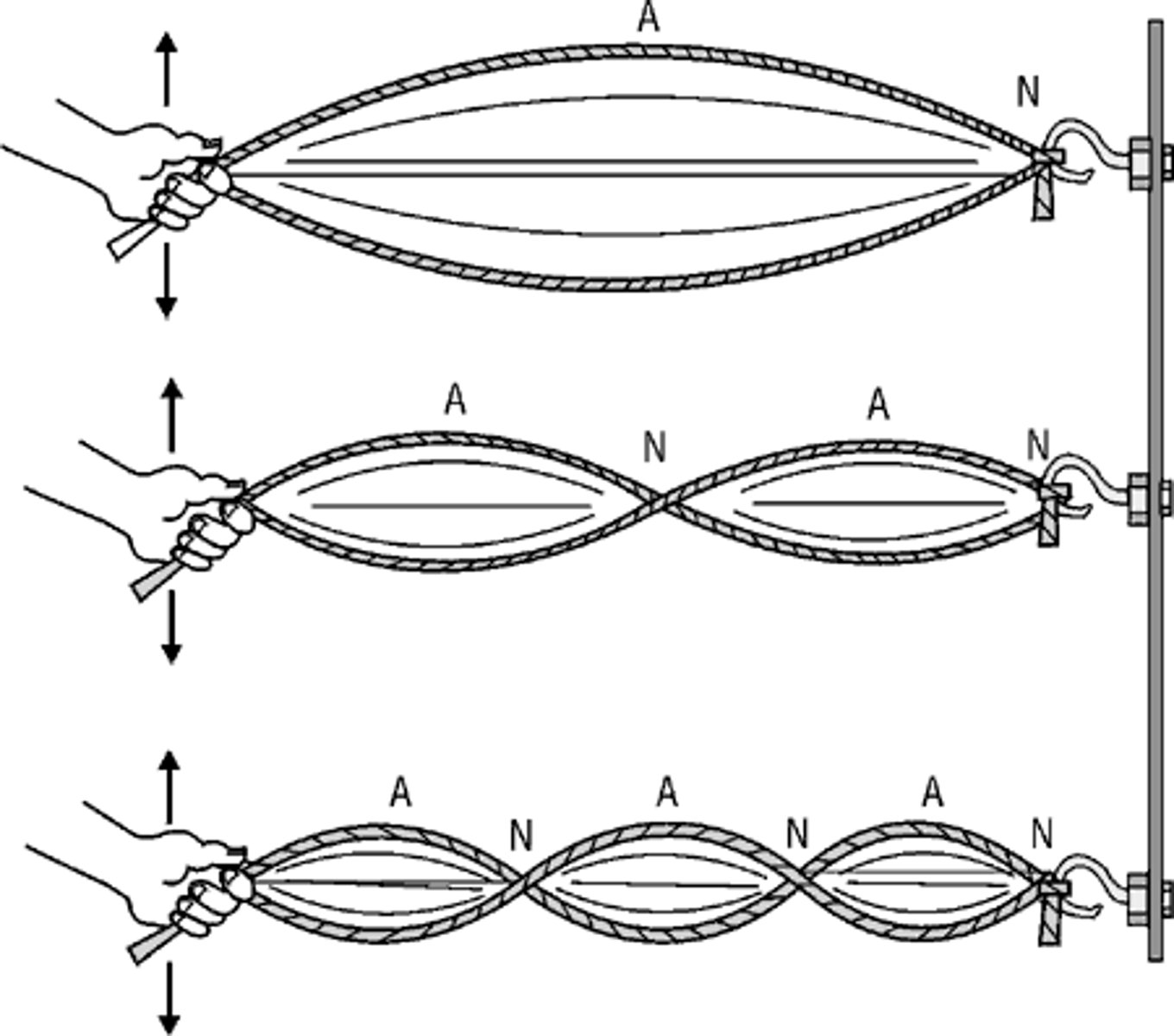
In Bohr's Model, each orbit has a specific _________ wavelength
integer
Heinsberg Uncertainty Principle
states that it is impossible to know both the position and velocity of an electron simultaneously
Orbital (what is it + how are they identified)
- region of space where the probability of finding an atom is high (represents the wave function squared, or Ψ^2)
- identified by a combination of three quantum numbers (n, l, ml)
Describe the meanings behind n, l, ml, and ms
- n = principal quantum number; indicates size + energy
- l = angular momentum number; defines orbital shape
- ml = magnetic quantum number; defines orientation
- ms = spin number; defines spin of electron
What values can n, l, ml, and ms have?
- n >= 1
- l: 0 to (n-1)
- ml: -l to l
- ms: +1/2 or -1/2
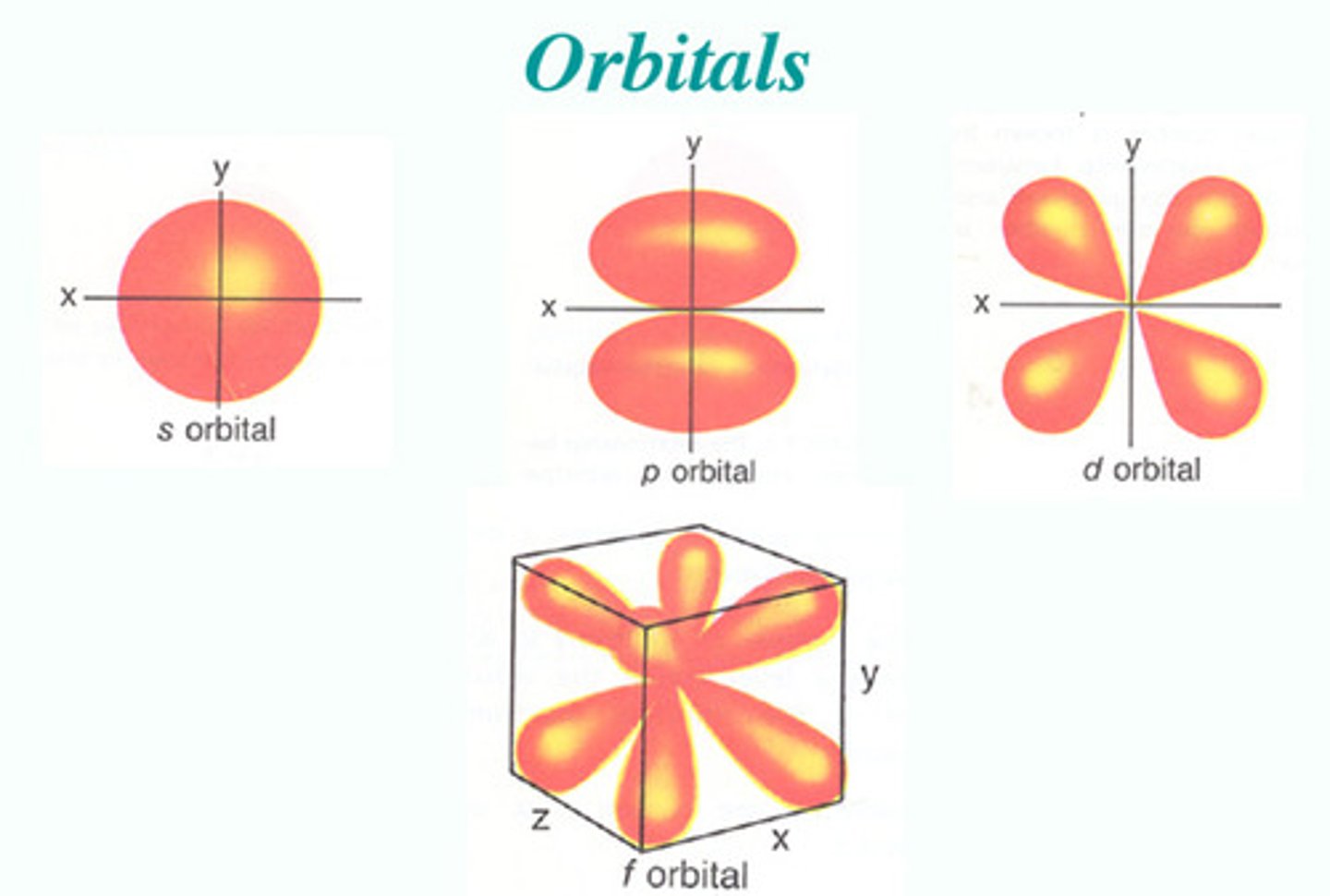
Define the values of l and the shapes they correspond to
l = 0 --> s (sphere)
l = 1 --> p (dumbbell)
l = 2 --> d (four-leaf clover OR dumbbell w/ doughnut shape in middle)
l = 3 --> f (complex)
Pauli Exclusion Principle (what is it and what did it help us realize?)
- states that no two electrons in the same atom can have the same set of quantum numbers
- proves that each orbital can only hold up to 2 electrons (one spin up, one spin down)
Isoelectronic
same electron configuration
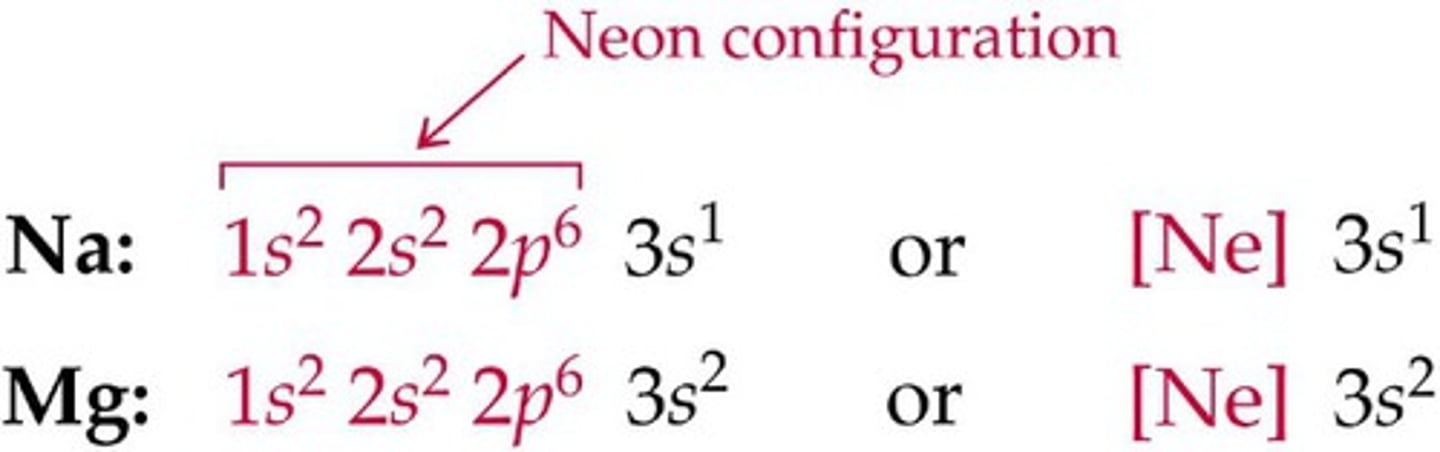
In electron configuration, anything in the outermost shell (highest n-value) represents the electrons in the _____________ ____________
valence shell
**in the image, the orbitals in black represent the valence shell
If the n and l values of two electrons are the same, that means they are in the same _________________ and are in _______________ orbitals (orbitals that have the same energy)
subshell, degenerate
The closer an orbital is the nucleus, the ___________ its energy is
lower
Aufbau Principle
the lower-energy orbitals are filled first
Hund's Rule
one electron must be in each degenerate orbital before they are paired up
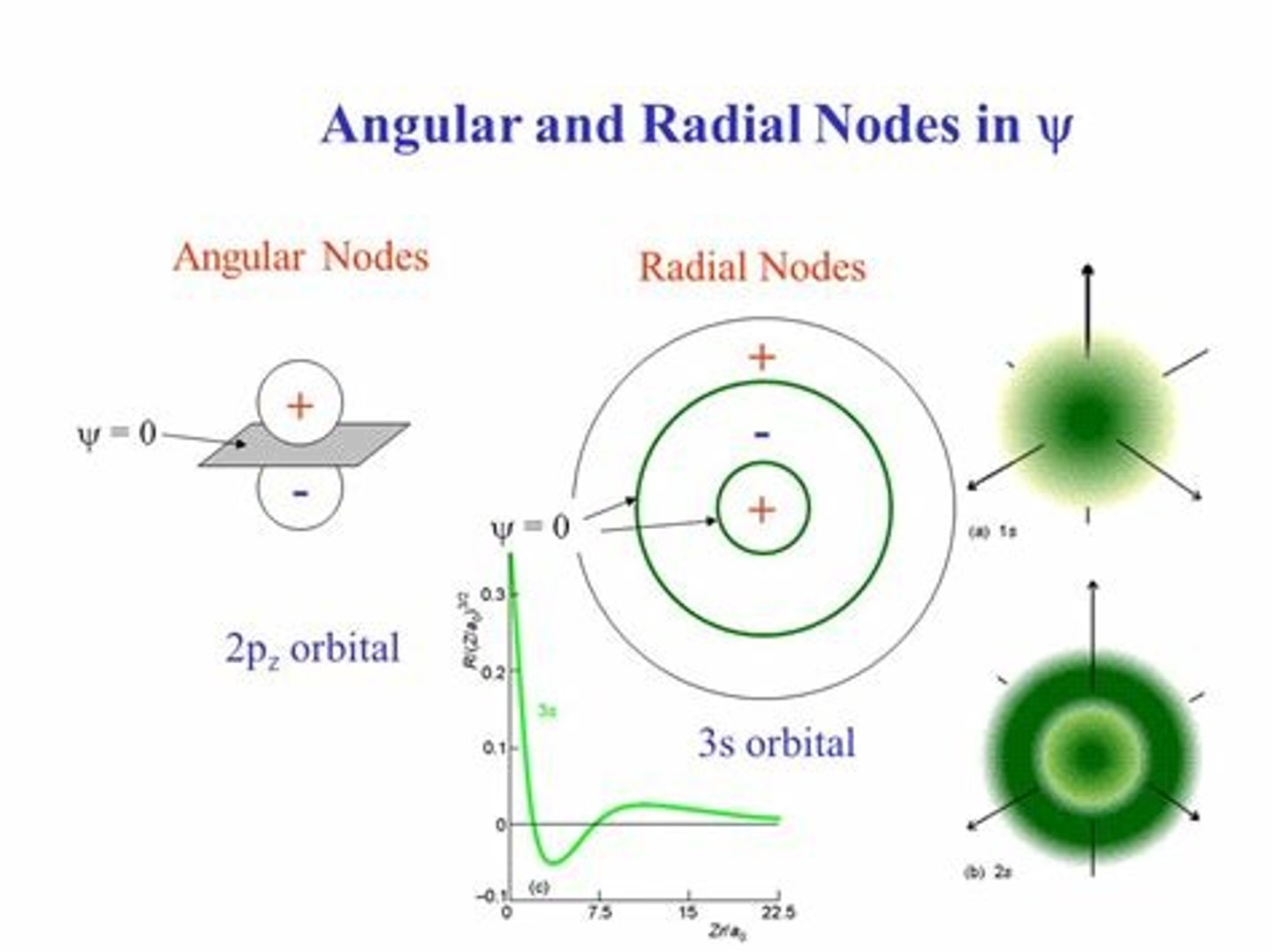
Radial Nodes vs. Planar Nodes (define what a node is + the different between these two types of nodes)
- Node: where there is zero electron density/areas where there is no probability of finding an electron
- Radial Nodes: spherical surface area where no electrons will be found (more common in the space between s orbitals)
- Planar Nodes: planes between orbitals where electrons will not be found (more common in space between p orbitals)