Carbohydrates and Lipids (copy)
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
Outline number of bonds carbon makes
4 nonpolar bonds
common functional groups
hydroxyl, carboxyl, phosphate, amine
4 major classes of carbon compounds
Lipids
DNA
Carbs
Proteins
Example molecules with branched chain, unbranched chain, single ring or multiple rings.
Rings: Carbs
Multiple rings: steroids
Branched Chain: hexane (CH6)
Unbranched Chain: methan (CH4)
Define Monomer and Polymer
Monomer: building block of polymer
Polymer: chains of monomers connected through bonds
Condensation reaction
When you add H2O to create a bond (reactant)
form of anabolism
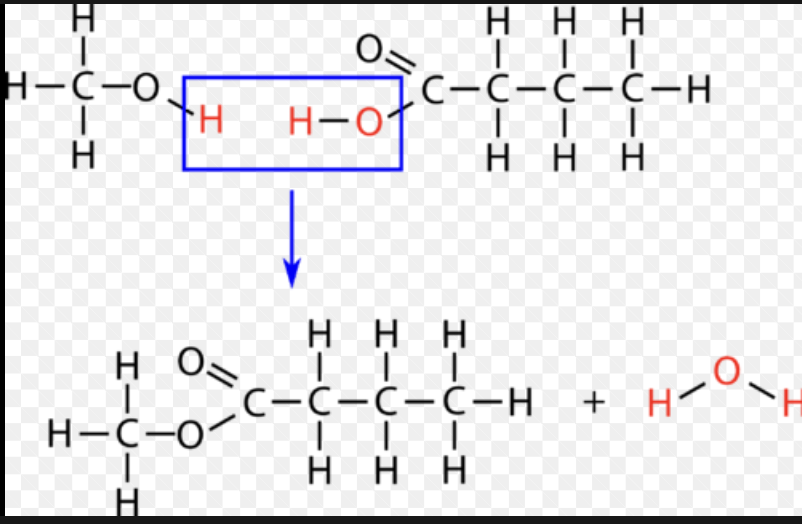
What is needed to produce macromolecules through reactions?
ATP
What is created through condensation reactions?
polypeptides, polysaccharides, nucleic acids
What happens in hydrolysis reactions?
type of catabolic reaction: breaks down polymers using water
water is used to spilt linked molecules
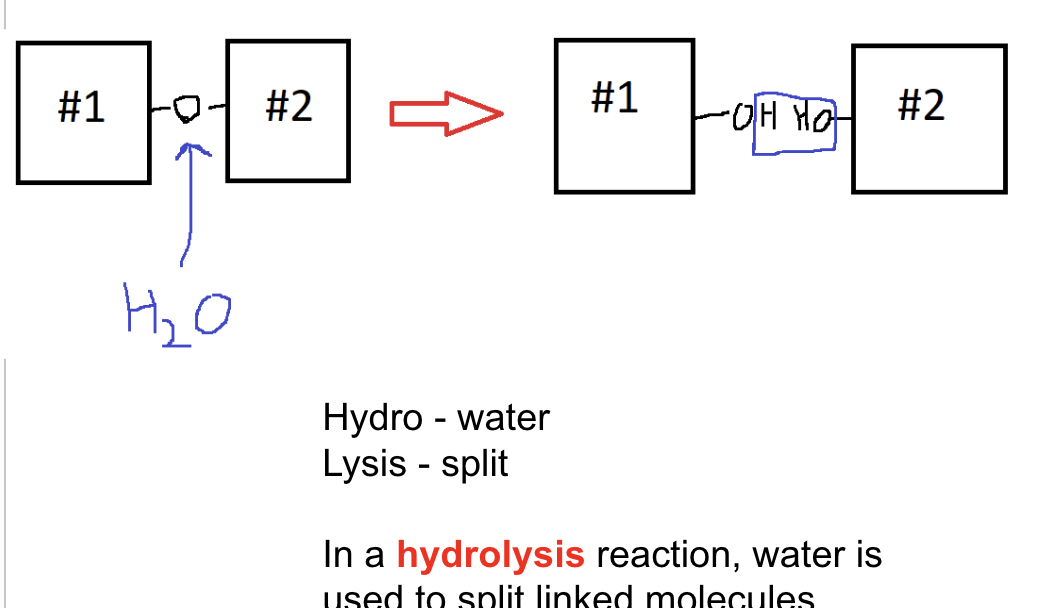
What is the difference between anabolic and catabolic reactions?
anabolic: building up
catabolic: breaking down
3 examples of anabolic processes
two monosaccharides joining through a glycosidic linkage
glycogen storage
muscle anabolism
DNA synthesis
3 examples of catabolic processes
protein catabolism: breaking down proteins to amino acids
breakdown of fat in adipose tissue
glycosis
digestive system breaking down food
cellular resp.
What is a monosaccharide?
monomer of carbohydrate
Backbone of 3-7 carbon atoms. Most common in biology are:
Pentoses = 5 carbon ring
Hexoses = 6 carbon ring
Form ring structures when dissolved in water (hence in cells form ring structures since cells are mostly water)
What are some properties of glucose?
solubility
good transportability
stability
energy yield from oxidation
why is glucose so soluble?
since it had many hydroxyl groups, it can form hydrogen bonds with water (polar dissolves in polar)
why is glucose transportable?
since it is soluble in water, glucose is also soluble in blood
how is glucose stable?
relatively stable. It does not readily undergo spontaneous chemical reactions, such as oxidation or hydrolysis, at a significant rate in the absence of enzymes.
explanation on how glucose has high energy yield
when oxidized, through cellular respiration glucose creates ATP that fuels many body processes
what is a polysaccharide?
Polymers of monosaccharides linked to form a chain. The chain may be branched or unbranched, and it may contain different types of monosaccharides.
amylose
comes from plants, form of starch, a- glucose, no branches
very coily and wrapped
function: to hydrolyze the glycosidic bonds in starch molecules, converting complex carbohydrates to simple sugars
starch that is consumed by humans is digested by enzymes, such as salivary amylases, into smaller molecules, such as maltose and glucose
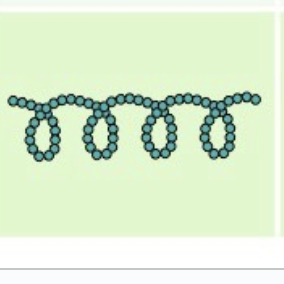
amylopectin
comes from plants, form of starch, a-beta glucose, branches
function: amylopectin has as its main function to store glucose for later use as an energy source
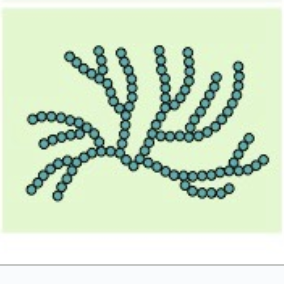
glycogen
storage form of carbohydrates in humans and other vertebrates.
comes from animals, a- glucose, branches
blood glucose levels increase (i.e. after eating), glucose is stored as glycogen
When blood glucose levels decrease, glycogen is digested to release glucose into the blood so the cells can continue doing cellular respiration.
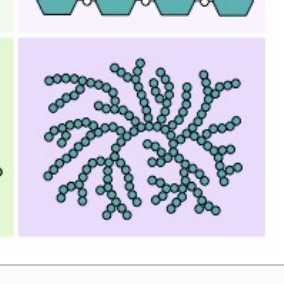
What is the benefit of polysaccharide coiling and branching during polymerization?
saves up space and makes it insoluble
How does condensation or hydrolysis of alpha-glucose monomers build or mobilize energy stores?
Glucose monomers are linked together through condensation reactions to form polysaccharides like starch (builds energy stores)
However, the hydroylsis of alpha glucose is seen here:
glycogen is digested to release glucose into the blood so the cells can continue doing cellular respiration.
and
digested by enzymes, such as salivary amylases, into smaller molecules, such as maltose and glucose. The cells can then absorb the glucose for use in cellular respiration.
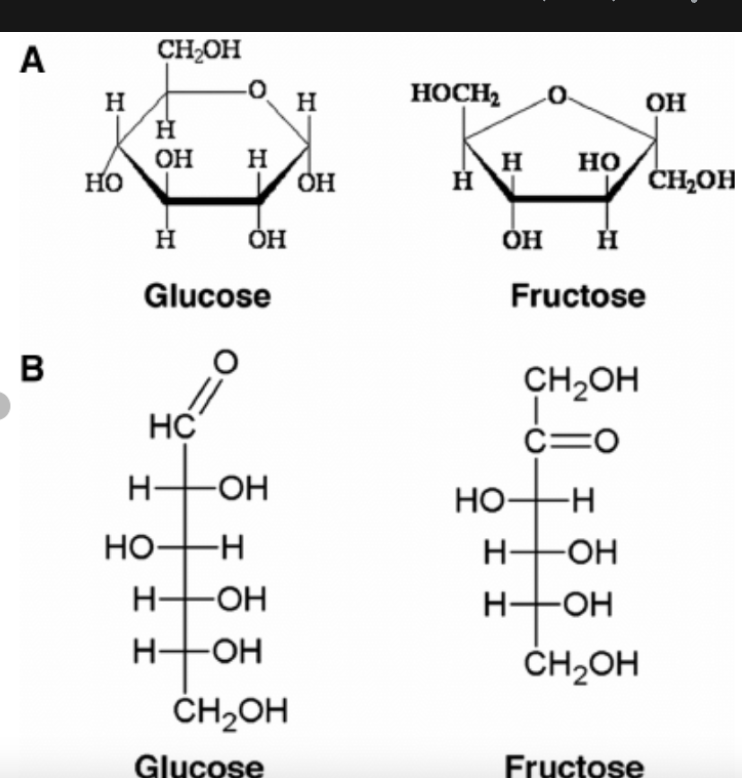
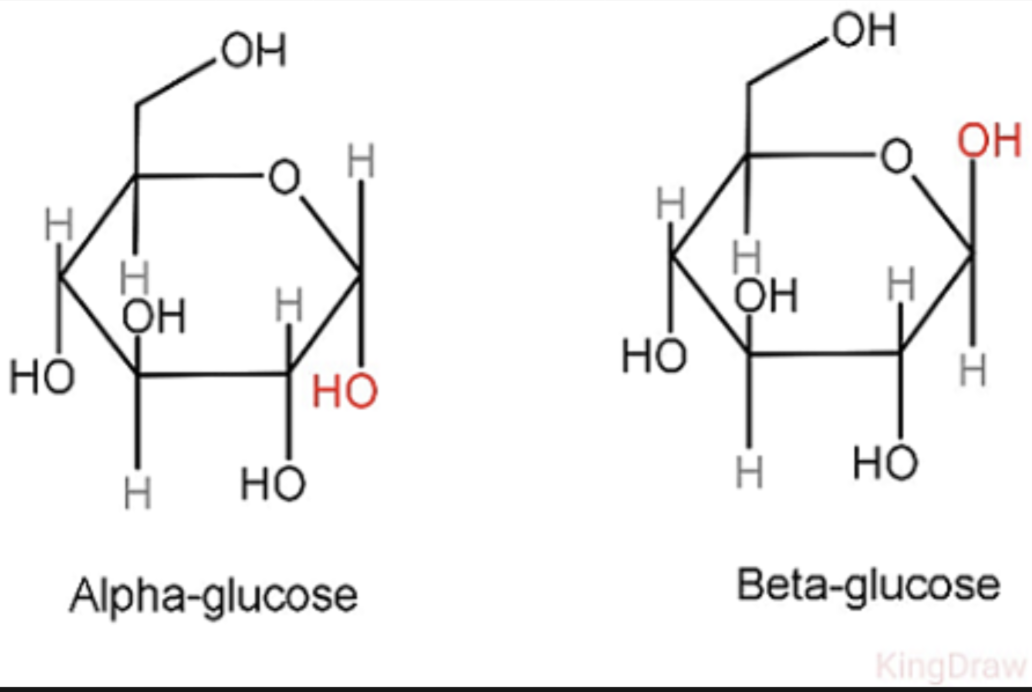
Compare the structure of alpha-glucose and beta-glucose.
alpha has hydroxyl on bottom, while beta is on top
Describe the structure of cellulose microfibrils.
Does not branch and packed in tight chains that form h.bonds and make cellulose stronger.
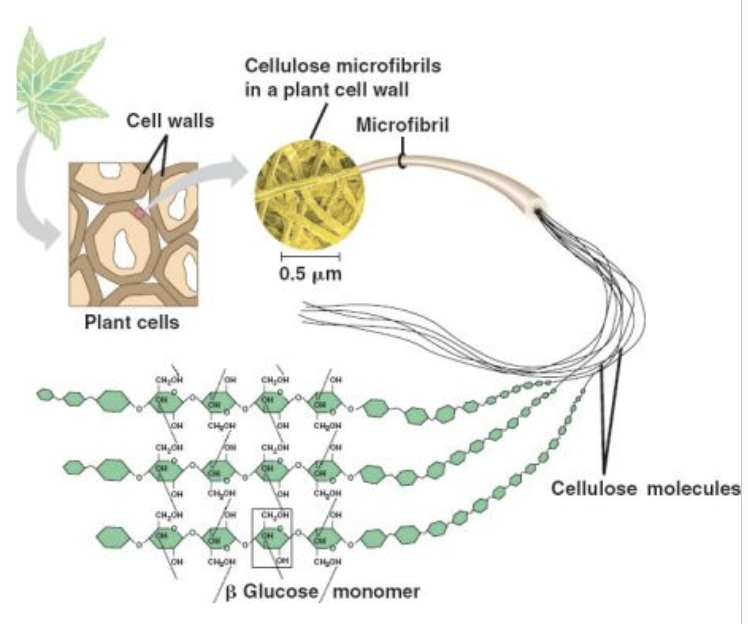
Discuss the consequences of the strength of cellulose in the plant cell wall.
this provides structural support to the cell.
Why are lipids hydrophobic?
Since lipids are non-polar, it cannot dissolve in water
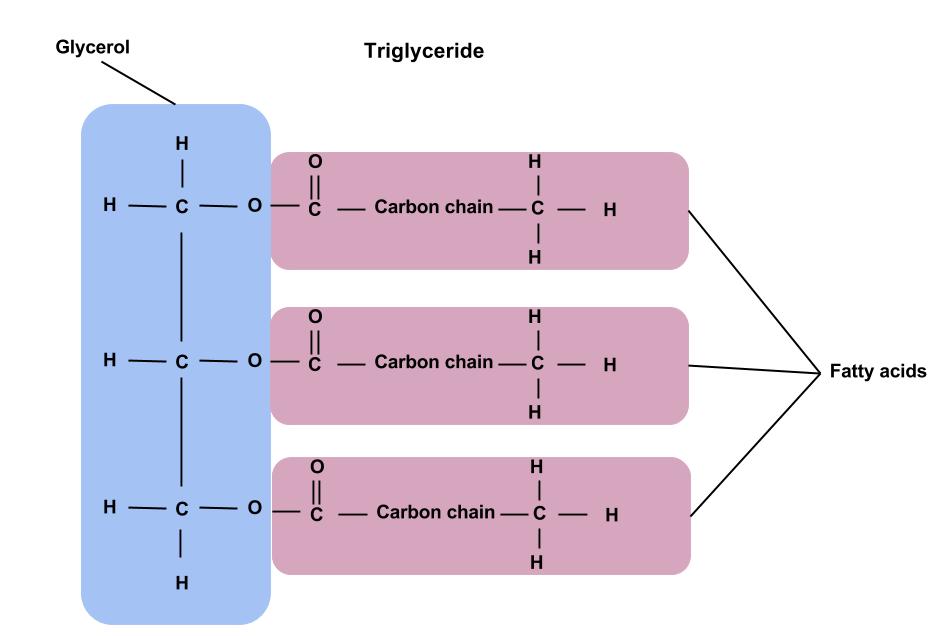
Outline the structure and function of fats and oils
Fats: solid at room temp
Oils: liquid at room temp.
Both store energy, insulate us and protect our vital organs.
Slower to build up and break down than carbs
Both triglycerides!: three fatty acids and one glycerol
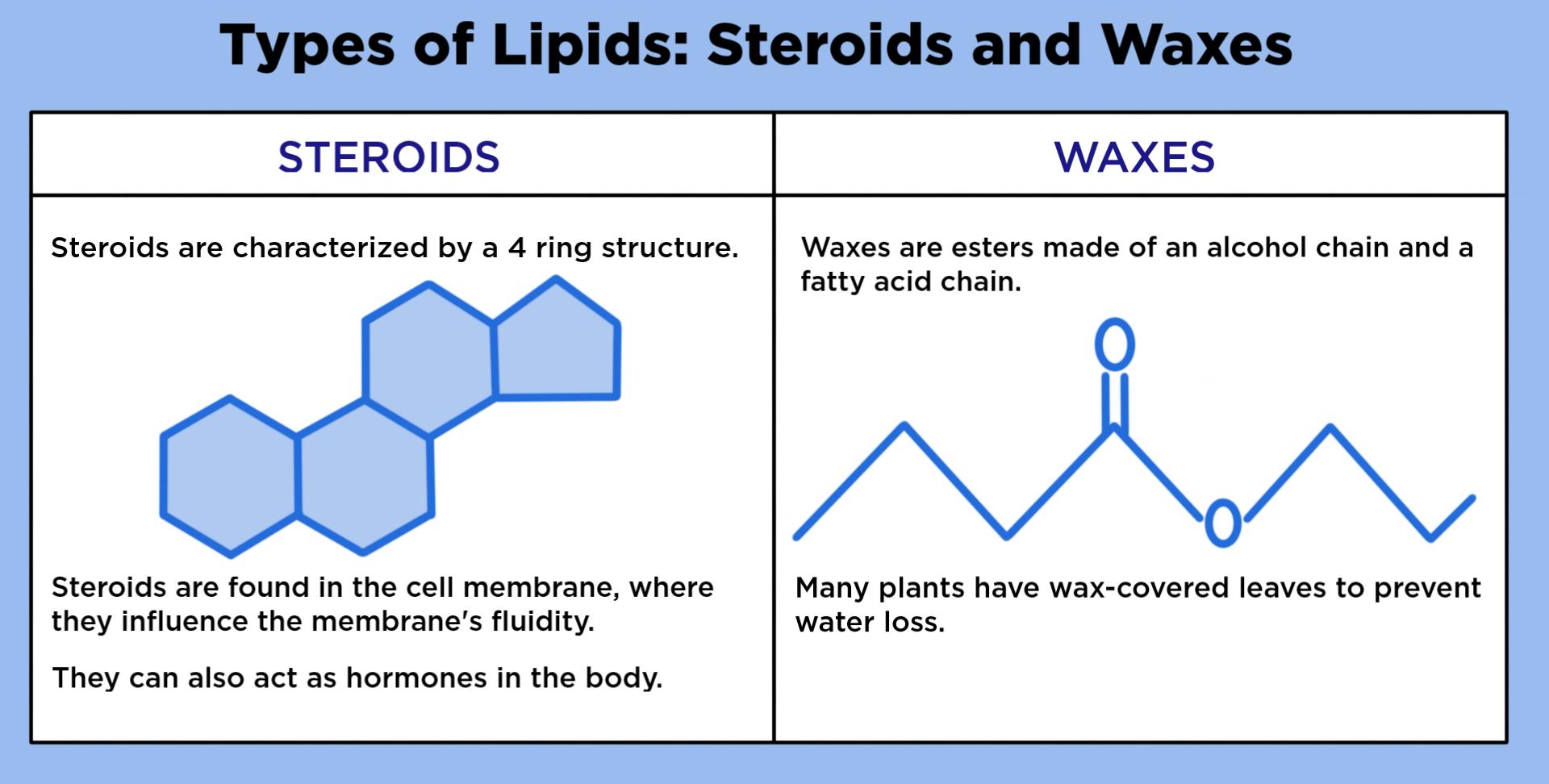
Outline the structure and function of waxes and steroids.
Waxes: Form waterproof structures and coatings
Composed of long hydrocarbon chains and are strongly hydrophobic
Highly saturated (few to no C=C)
Solid at room temperature
Steroids: multiple rings tied together and are chemical messangers in endocrine system
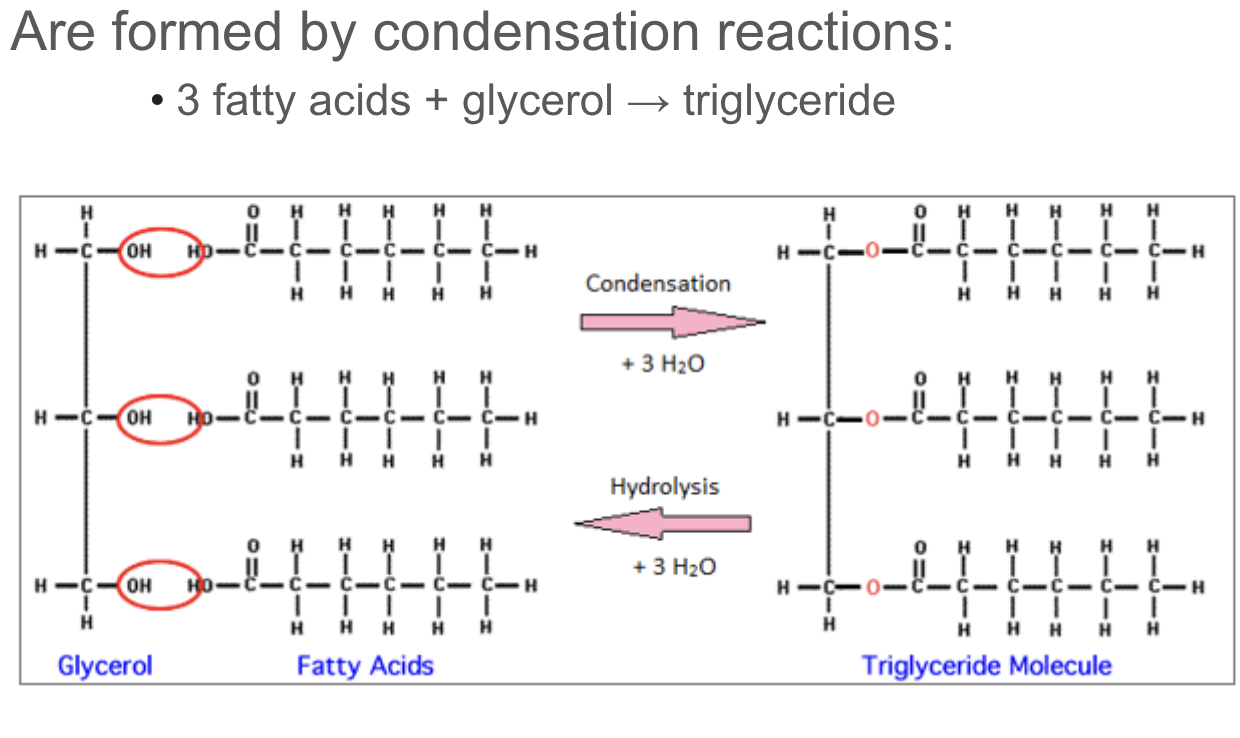
Explain the condensation reaction connecting fatty acids and glycerol to form a triglyceride.
glycerol has three pairs of hydroxyl groups while the fatty acids have hydrogen. these two things combine in a condensation reaction and water is produced to bind them together
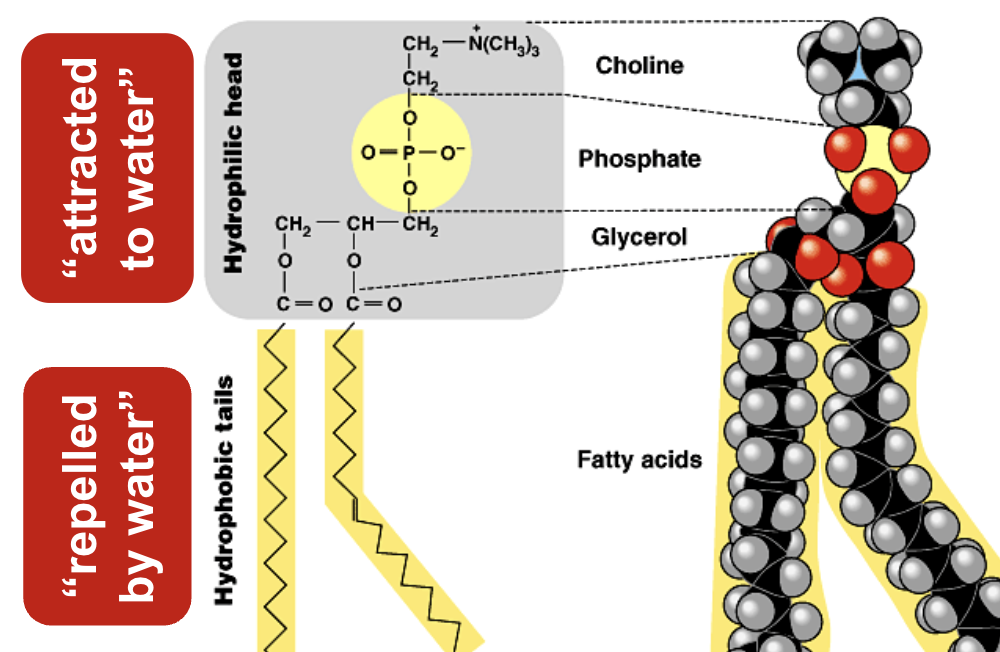
Explain the condensation reaction connecting fatty acids, glycerol and a phosphate group to form a phospholipid.
Formed by attachment of two fatty acids plus a phosphate group to a glycerol.
structure of a generalized fatty acid
long hydrocarbon chain of varying length
with a COOH group (“carboxyl”) at one end
and a CH3 (“methyl”) group at the other end.
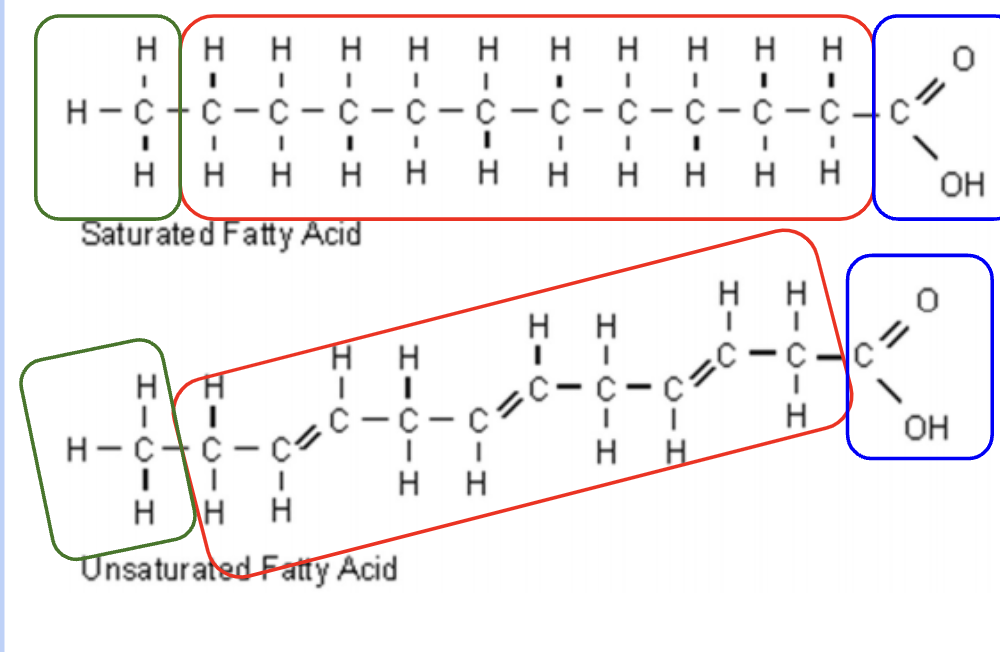
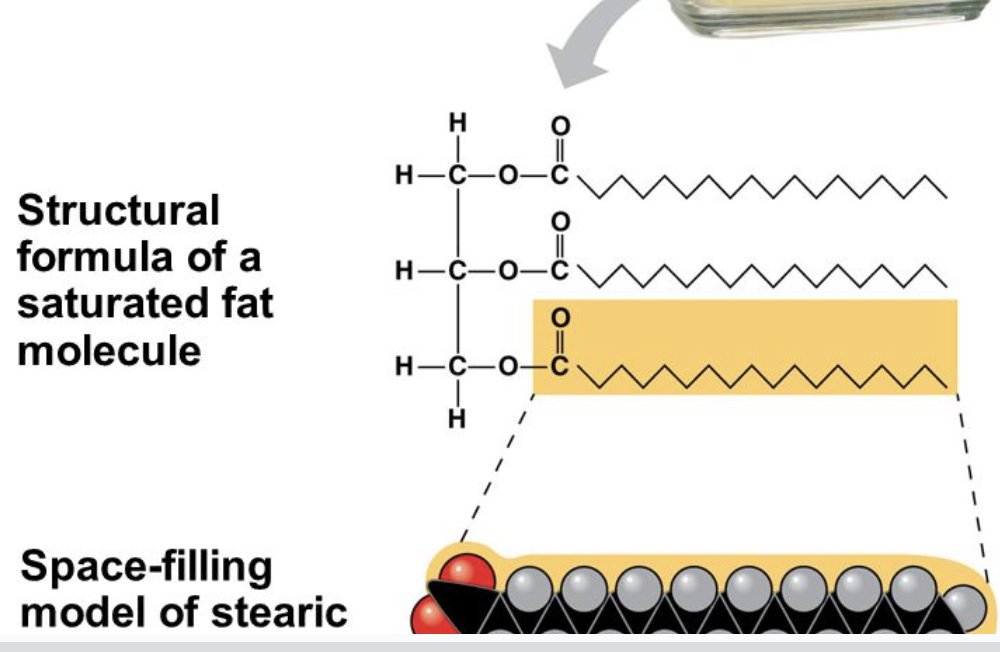
Saturated fatty acids
single bonds only
solid at room temp and come from animal sources
very straight
Since the fatty acids are straight, the fat molecules can pack together tightly, forming a solid at room temperature
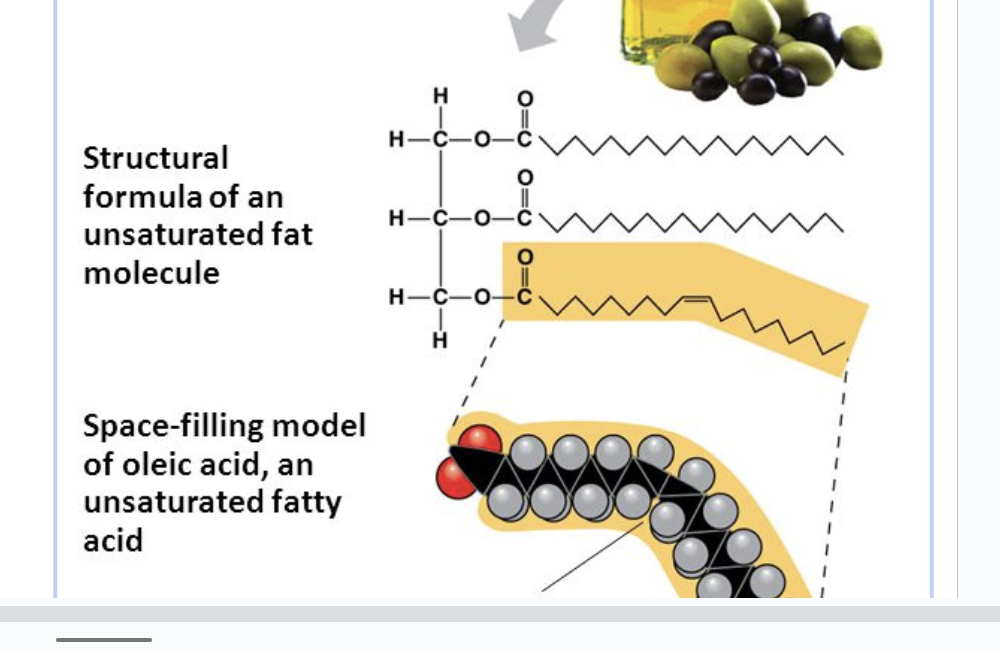
Unsaturated fatty acids
double bonds can be seen
liquid at room temp and are often from plants
the double bonds in the fatty acids creates bends in the molecule. These can’t pack together as tightly, resulting in a lower melting point.
State the function of adipose tissue.
Adipose tissue, otherwise known as body fat, is a connective tissue that extends throughout your body
insulates the body from extreme temperatures, cushions vital organs, and secretes hormones and biological factors
Discuss the adaptation of a thick adipose tissue layer as a thermal insulator.
low thermal conductivity, which means that it does not transfer heat as well as other tissues and materials—such as muscle or skin. That way, it helps to insulate an animal's body. (fat storing tissue)
Outline properties of triglycerides that make them suitable for long-term energy storage.
low water content: since water doesn’t have to triglycerides, more energy can be compacted with ease
high energy density- twice as much energy compared to carbs (9 to 4)
slow metabolism- energy cannot be released
insulation and protection- stored in adipose tissue and this tissue protects organs and maintains energy
lightweight storage- stored in lipid droplets that take up little cellular space
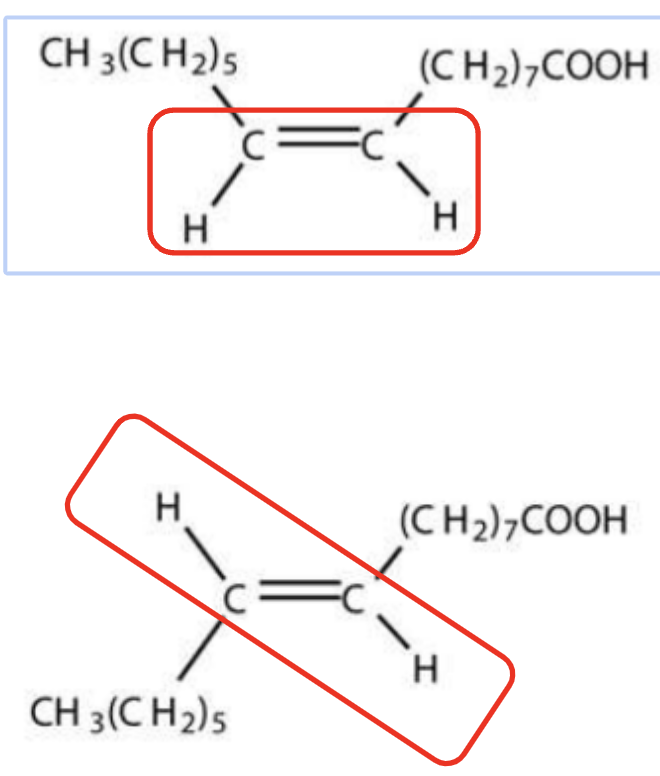
Distinguish between the structure and properties of cis- and trans-unsaturated fatty acids
Cis: Naturally occurring
Hydrogen atoms are "on the same side” of the C=C bond
Causes a kink in the fatty acid chain
Trans:
Are not found in nature and are the result of human food processing
Hydrogen atoms are "on the opposite side" of the C=C bond
Causes a straight(er) fatty acid chain
top is cis, bottom is trans