AP Biology - unit 1
5.0(1)
Card Sorting
1/120
Earn XP
Last updated 11:51 AM on 11/14/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
121 Terms
1
New cards
How does carbon enter the biosphere?
Plants use solar energy to transfer carbon dioxide into the molecules of life such as carbon.
2
New cards
Why is the tetra valence of carbon important?
It gives carbon the ability to form large complex and varied molecules.
3
New cards
What are organic compounds?
Carbon containing compounds.
4
New cards
What is organic chemistry?
It is the study of carbon containing compounds.
5
New cards
What are hydrocarbons?
They are compounds containing the elements hydrogen and carbon.
6
New cards
What is vitalism?
It is the belief of a life force outside the jurisdiction of physical and chemical laws.
7
New cards
What is mechanism?
The belief that all natural phenomena are governed by physical and chemical laws including the process of life.
8
New cards
What and how did Stanley Miller conclude in 1953?
He mimicked the conditions of early earth and ended up with Abiotically synthesized organic compounds such as ammonia and methane.
9
New cards
Is CO2 an organic compound? why?
Carbon dioxide is not an organic compound because it is very simple and does not contain hydrogen.
10
New cards
What are the carbon skeleton variations?
carbon skeletons can be in branches closed rings or chain. Carbon atoms can also form single triple or double bonds with each other.
11
New cards
What are the properties of hydrocarbons?
They are hydrophobic because of their nonpolar linkages, they can undergo reactions and release large amounts of energy.
12
New cards
What are isomers?
They are compounds that have the same number of atoms of the same element with different structures and therefore different functions.
13
New cards
What are the three types of isomers?
Structural isomers, geometrical isomers, enantiomers.
14
New cards
What are structural isomers?
They differ in covalent arrangement (carbon skeleton) and double bond locations.
15
New cards
What are geometric isomers?
They differed in the arrangement about a double bond. ( Cis when H’s are next to each other and trans when H’s are opposite each other) (only with double bonds)
16
New cards
What are enantiomers?
They are emitted images of each other due to the presence of an asymmetric carbon.
17
New cards
How many isomers of a compound are biologically active?
One, because only that form can find to specific molecules in an organism.
18
New cards
How do chemical groups affect the functioning of biological molecules?
By changing the molecules shaped or by being involved in chemical reactions.
19
New cards
What are functional groups?
They are chemical groups that are directly involved in chemical reactions.
20
New cards
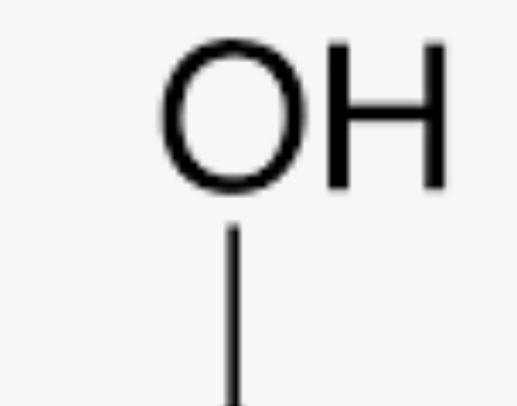
Hydroxyl group
-OH or HO- (Forms alcohols) (hydrophilic and increases compound’s solubility in water)
21
New cards
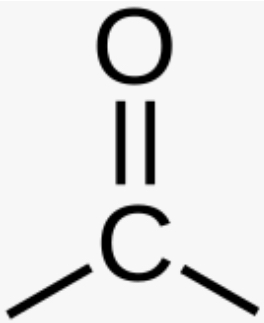
Corbonyl group
>CO (forms ketones and aldehydes which for ketoses and aldoses sugars) (hydrophilic and increases compound’s solubility in water)
22
New cards
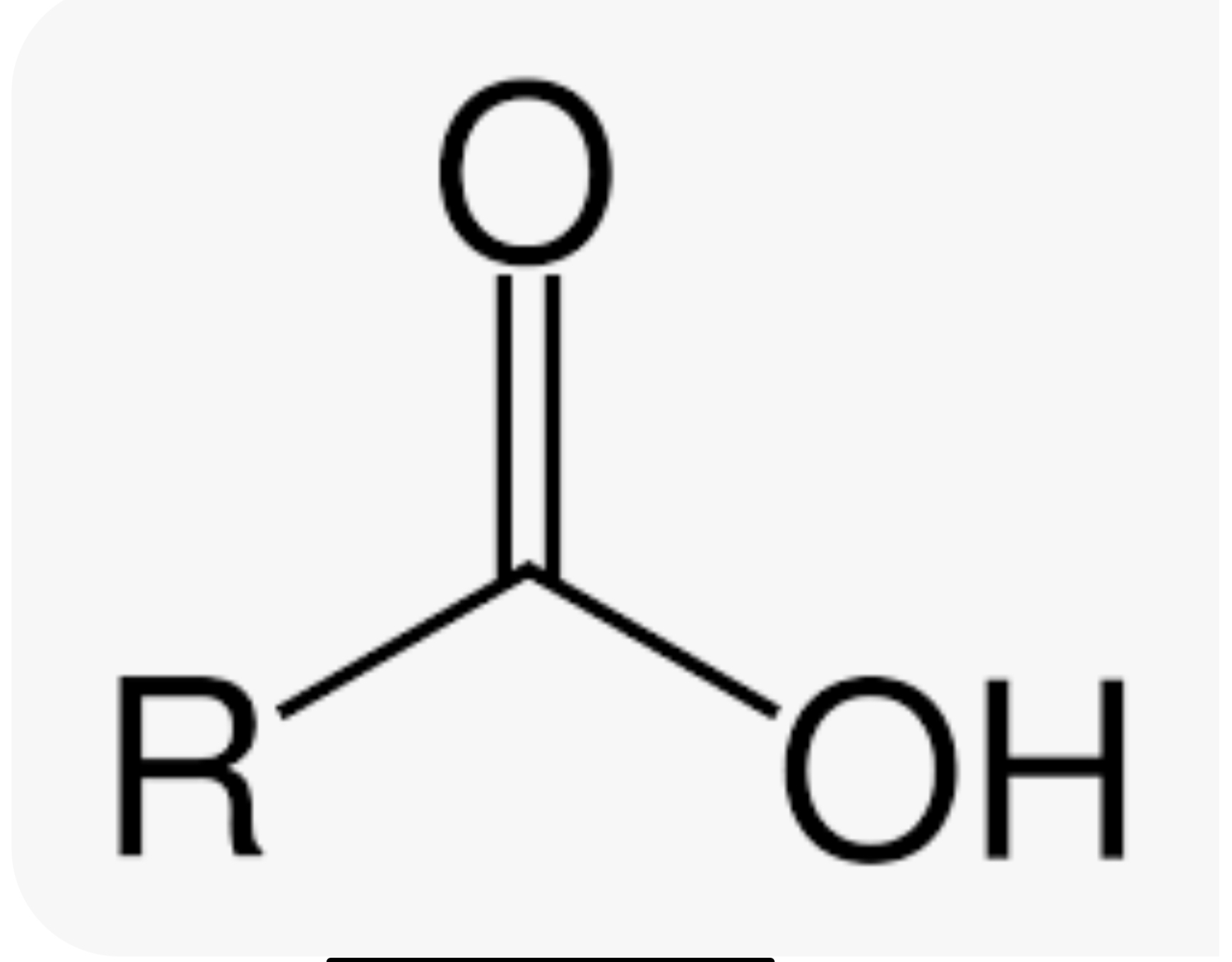
Carboxyl group
-COOH (forms carboxylic acids)(hydroxyl + carbonyl) (hydrophilic and increases compound’s solubility in water) (acts as an acid)
23
New cards
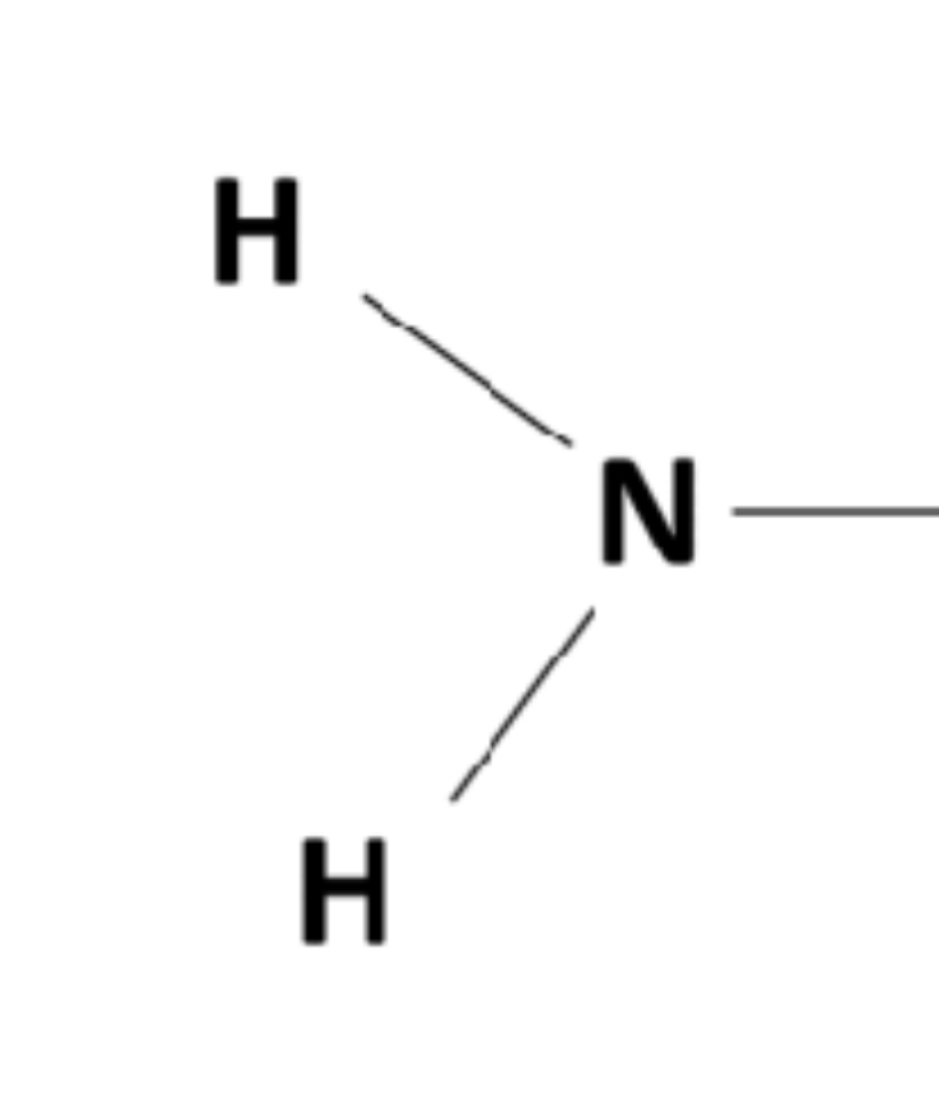
Amine group
-NH2 (forms amines) (acts as a base) (hydrophilic and increases compound’s solubility in water)
24
New cards
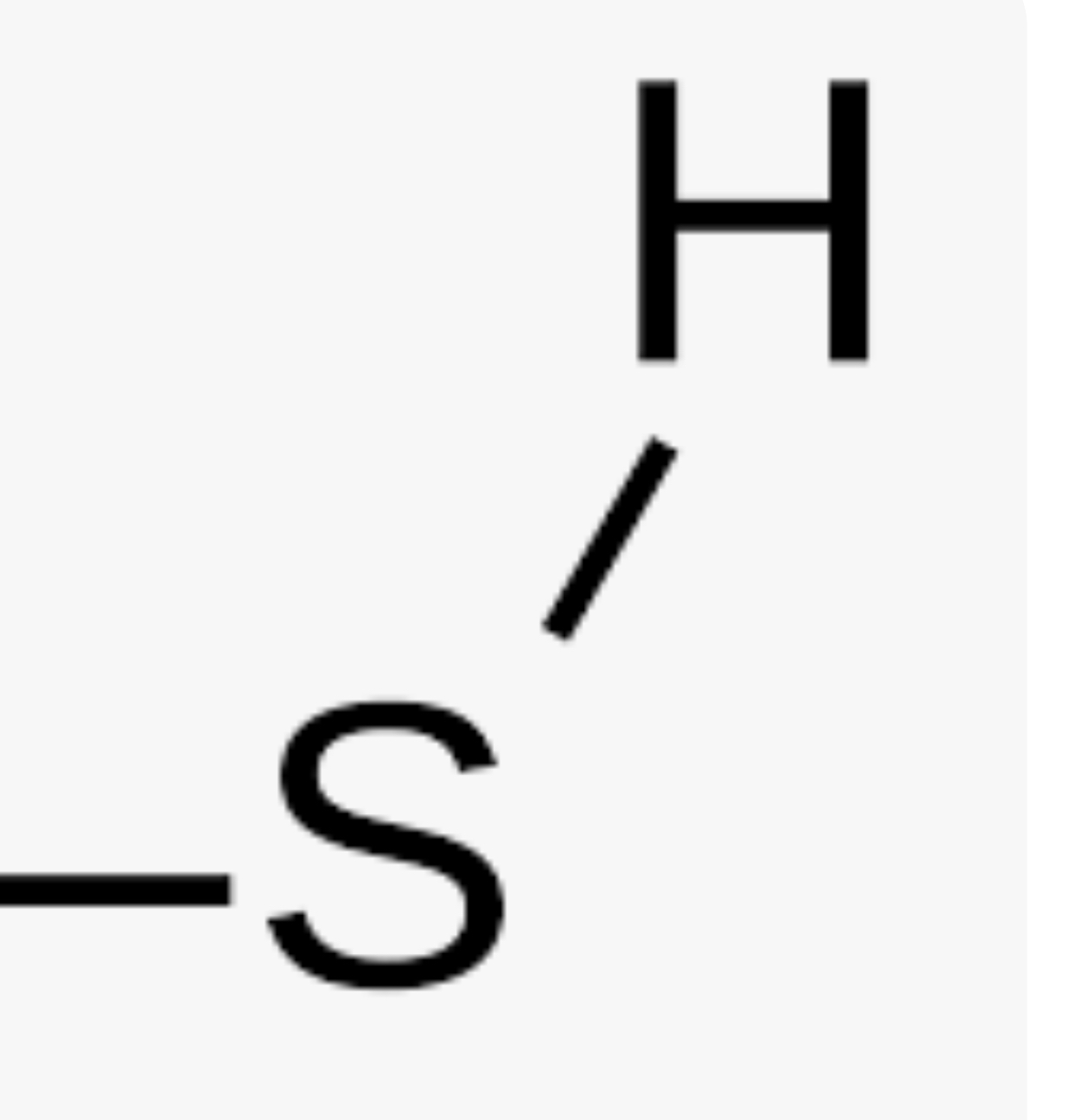
Sulfhydrl group
-SH or HS- (forms thiols) (hydrophilic and increases compound’s solubility in water)
25
New cards
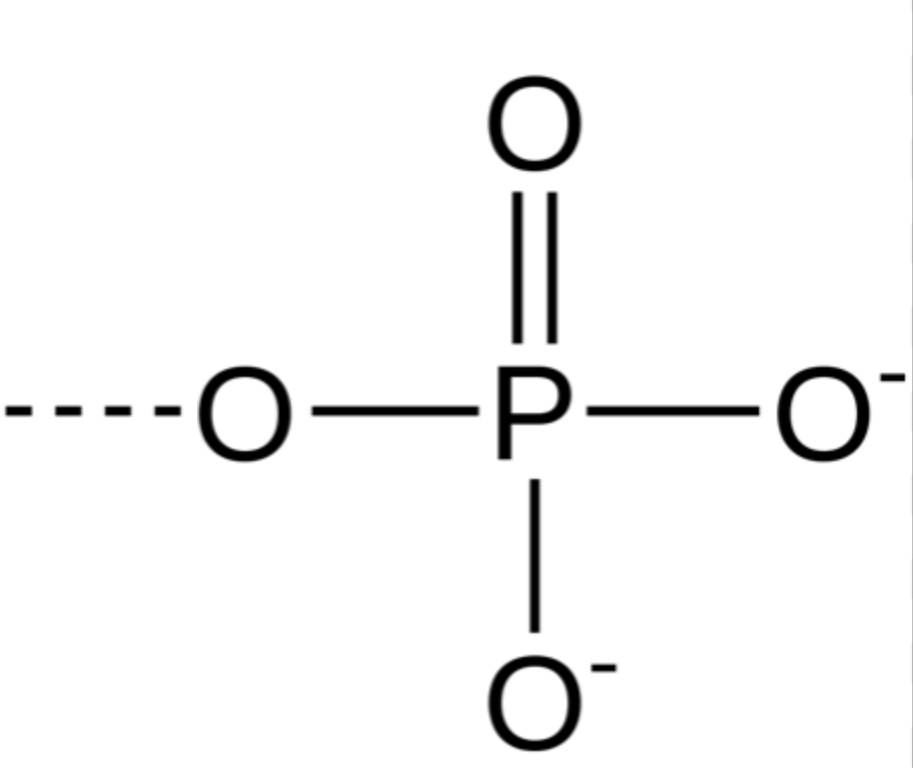
Phosphate group
-OPO3^2- (forms organic phosphates) (hydrophilic and increases compound’s solubility in water)
26
New cards
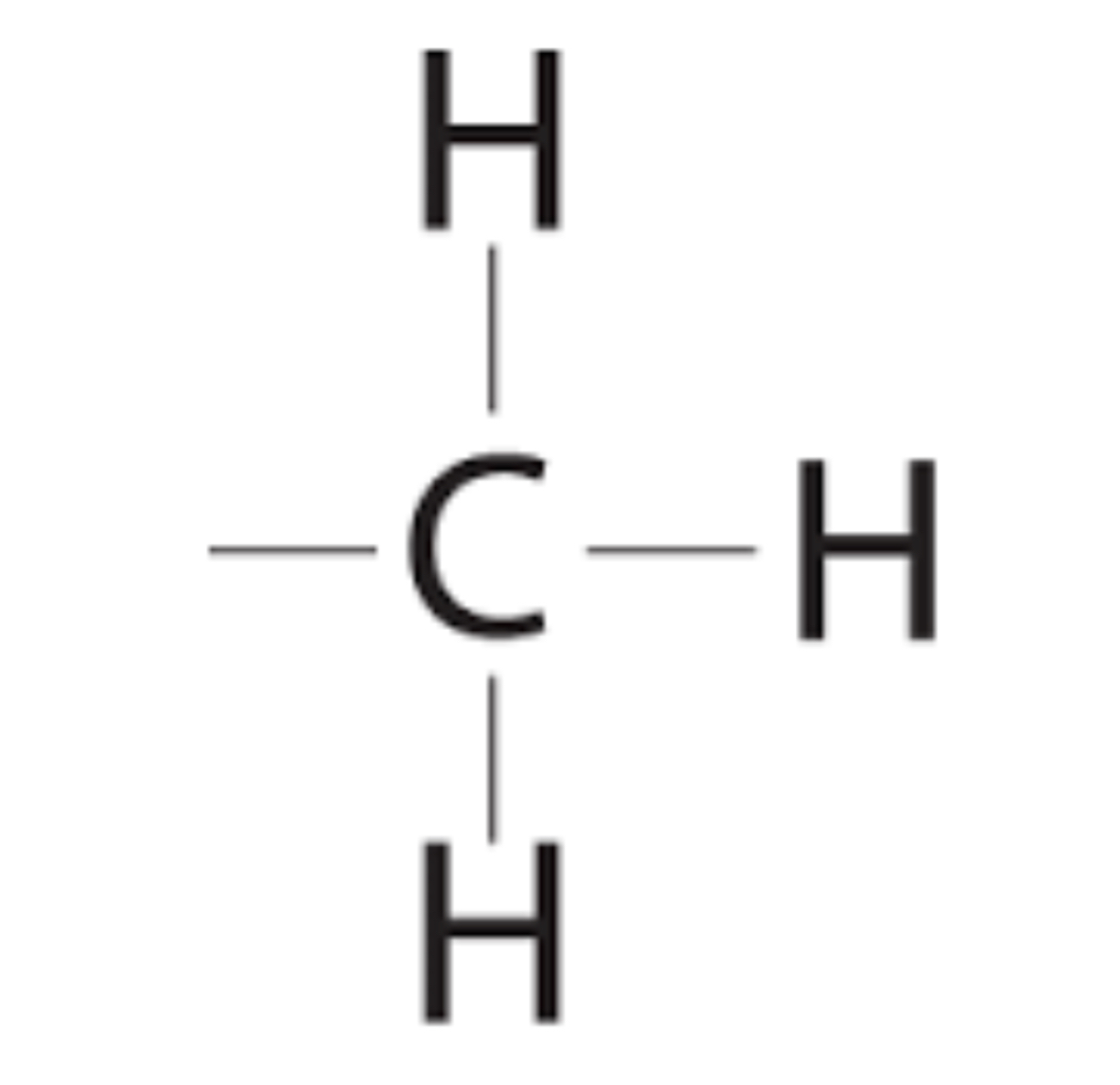
Methyl group
-CH3 (forms methylated compounds) (does not react but acts as a tag on molecules)
27
New cards
What is ATP composed of?
Adenosine attached to a string of 3 phosphate groups. It can react with water and split of into ADP and a phosphate group and release energy for cells.
28
New cards
How many classes of macromolecules are critically important for living things?
Four: lipids, proteins, carbohydrates, nucleic acids.
29
New cards
Why are they called macromolecules?
because they are huge on the molecular scale.
30
New cards
What are polymers?
A polymer is a long molecule consisting of many similar blocks linked by covalent bonds.
31
New cards
What are monomers?
They are the building blocks of polymers.
32
New cards
What are enzymes?
They are specialized macromolecules that speed up chemical reactions.
33
New cards
What reaction helps in the assembly of monomers?
Dehydration synthesis/condensation reaction.
34
New cards
What is a dehydration reaction?
It is a reaction in which two molecules are covalently bonded with the loss of one water molecule in which one monomer provides a hydroxyl group and the other monomer provides a hydrogen.
35
New cards
Which reaction helps the disassembling of polymers?
Hydrolysis reaction.
36
New cards
What is a hydrolysis reaction?
It is a reaction in which the bonds between monomers is broken down by the addition of water in which a hydrogen attaches to one monomer and a hydroxyl group attaches to the other monomer.
37
New cards
What is the basis for diversity in life‘s polymers?
The basis is that small molecules common to all organisms are ordered into unique macromolecules.
38
New cards
What are carbohydrates?
They are sugars are polymers of sugar and
act As fuel and building material.
act As fuel and building material.
39
New cards
What are monosaccharides?
They are simple sugars and monomers of complex carbohydrates.
40
New cards
What are disaccharides?
They are double sugars consisting of two covalently bonded monosaccharides.
41
New cards
What are polysaccharides?
They are polymers of a few hundreds or thousands of monosaccharides.
42
New cards
What is the emerical formula of monosaccharides?
CH2O
43
New cards
What are the functional groups in a monosaccharide?
One carbonyl group and multiple hydroxyl groups.
44
New cards
How long are carbon chains in monosaccharides?
They range from 3 to 7 carbons. The most common ones are trioses, pentoses, and hexoses. They are arranged in rings.
45
New cards
What are the sources of diversity for simple sugars?
The location of carbonyl group, the spatial arrangement of their parts around asymmetric carbon‘s, and of the length of the carbon chains.
46
New cards
What is a glycosidic linkage?
It’s a covalent bond formed between two monosaccharides by dehydration synthesis.
47
New cards
What determines the architecture and function of a polysaccharide?
The locations of glycostatic linkages and sugar monomers.
48
New cards
What are the storage polysaccharides?
Starch and glycogen.
49
New cards
What is starch?
It is a polymer of glucose used to store sugars in plants in plastids. Sugar can be withdrawn by hydrolysis.
50
New cards
What is the difference between amylose and amylopectin?
Amylose is unbranched and is the simplest starch. Amylopectin is branched and more complex.
51
New cards
What is glycogen?
An extensively branched polymer of glucose that stores is glucose in animals in liver and muscle cells. It cannot sustain animals for long.
52
New cards
What are the structural polysaccharides?
Cellulose and chitin.
53
New cards
What is cellulose?
It is a polymer of glucose that makes up plant cell walls. It is the most abundant organic compound on earth.
54
New cards
What is the difference between glycol Citic linkage is in cellulose and starch?
Sarge is made up of alpha glucose monomers (OH is under carbon 1). Cellulose is made up of beta glucose monomers (OH is above carbon 1).
55
New cards
What is the structure of cellulose‘s carbon skeleton?
It is always straight and not very branched. Some hydroxyl groups are free to hydrogen bond with hydroxyl groups of other cellulose molecules to form microfibrils.
56
New cards
Why is cellulose important for humans?
Although cellulose does not nutrient for humans, it’s a bridge the walls of digestive tract and stimulates mucus for smooth passage of food.
57
New cards
How are cellulose and starch monomers oriented?
Starch monomers are in the same orientation while each cellulose monomer is upside down its neighbors.
58
New cards
What is chitin?
A polymer of glucose monomers that is used in exoskeletons of arthropods and cell walls of fungi.
59
New cards
What is the texture of pure chitin?
Pure chitin is levity and flexible, in exoskeletons it is encrusted with calcium carbonate
60
New cards
What is the difference between chitin and cellulose?
Glucose monomers in chitin have nitrogen containing appendages.
61
New cards
Do lipids form polymers?
Lipids do not form true polymers and they are not large enough to be considered macromolecules.
62
New cards
Why are lipids grouped together?
Because of their hydrophobic properties which come from their many hydrocarbon regions.
63
New cards
What are three types of lipids?
Fats, phospholipids, and steroids.
64
New cards
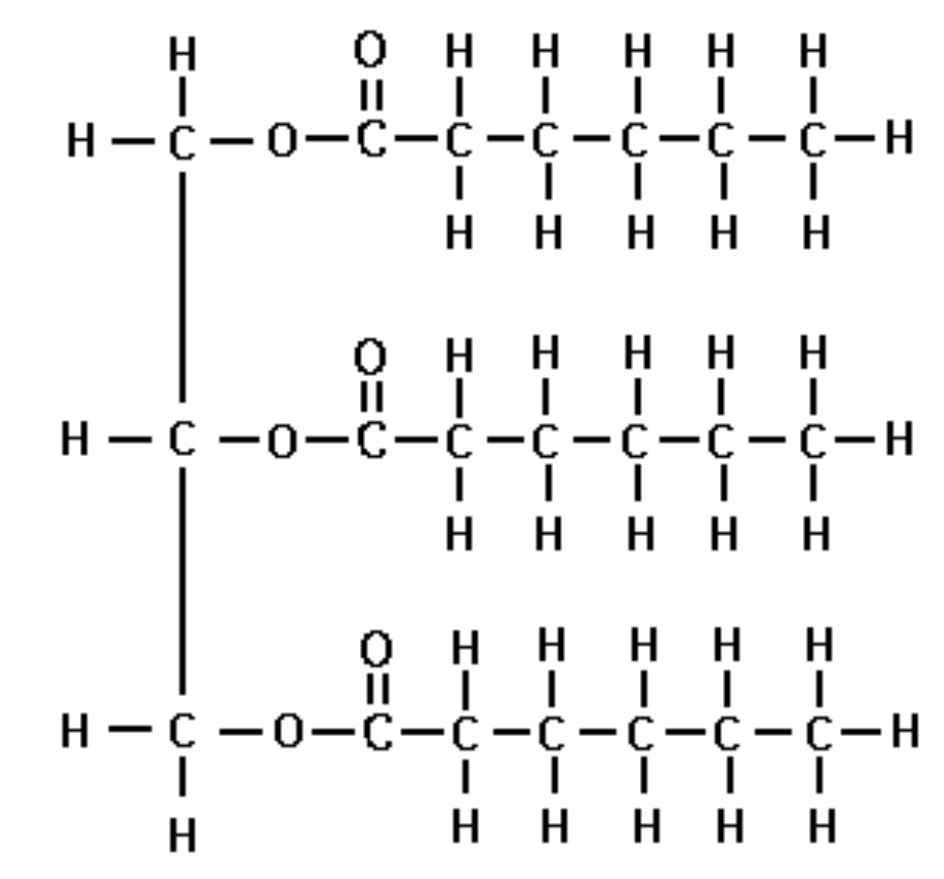
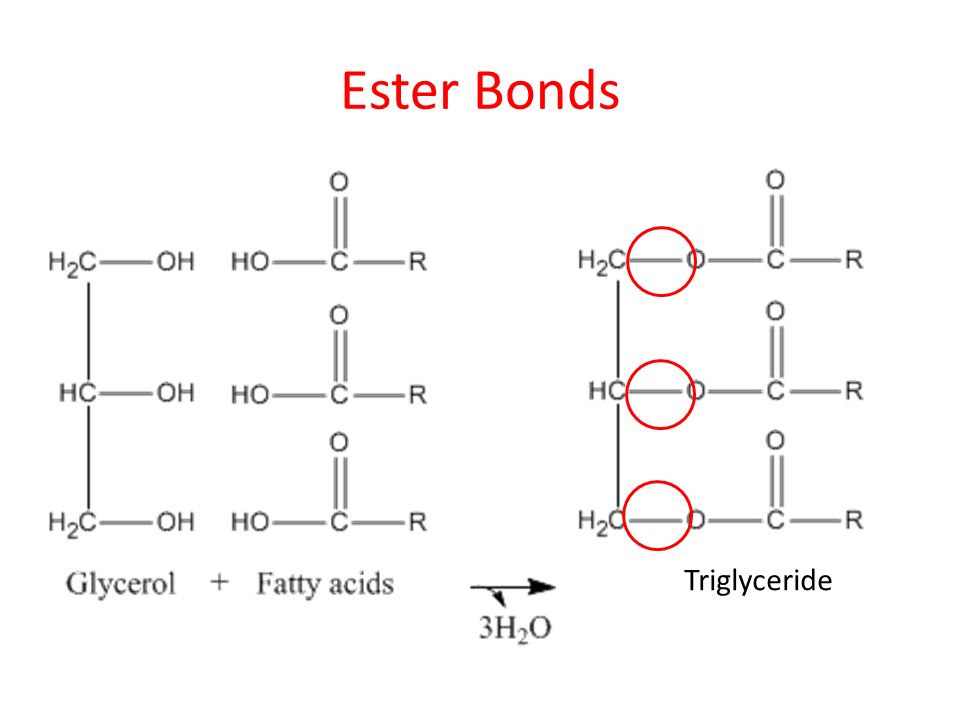
What are the components of a fat?
A fat molecule is composed of three fatty acids (can be the same or different kinds) that are joint to glycerol by an ester linkage.
65
New cards
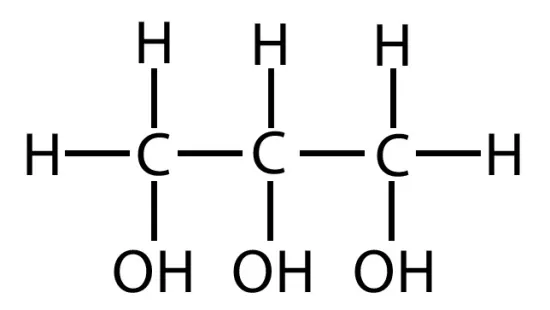
What is glycerol?
Glycerol is an alcohol whose each of its three carbos bears a hydroxyl group.
66
New cards
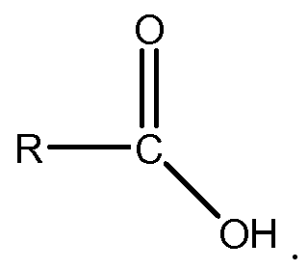
What is fatty acid?
It is a long carbon chain (usually 16 or 18)with carboxyl group at one end which gives it the name fatty acid.
67
New cards
What is an ester linkage?
It is a bond between a hydroxyl and carboxyl group
68
New cards
What are the other names of fats?
Triacylglycerol and triglyceride.
69
New cards
What is a saturated fatty acid?
Fatty acids with no double bonds between carbons and therefore with as many hydrogen atoms as possible.
70
New cards
What are unsaturated fatty acids?
Fatty acids with at least one double bond between carbons (mostly cis double bond which causes a up curve in the hydrocarbon)
71
New cards
What are the properties of saturated fats?
Animal fats - solid at room temperature (their flexibility due to lack of double bonds allows them to tightly pack) - contribute to cardiovascular diseases (by raising cholesterol levels)
72
New cards
What are the properties of unsaturated fats?
Plant fats - fish fat - nut fat - liquid at room temperature( kinks from cis bonds prevents packing tightly) - referred to as oils.
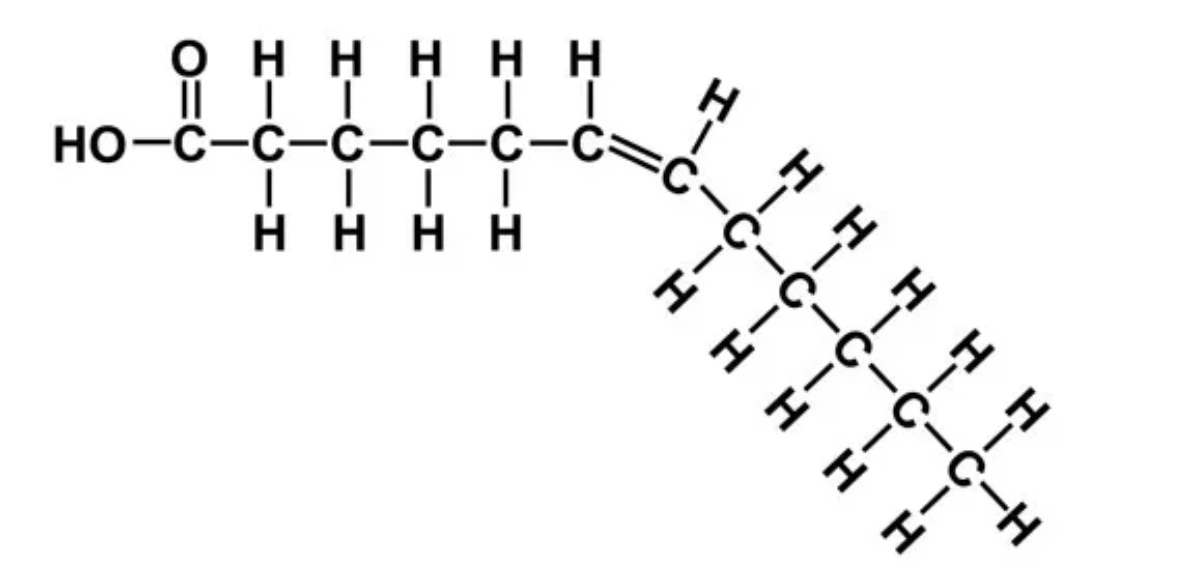
73
New cards
What is hydrogenating unsaturated fats?
Hydrogenating oils is synthetically converting them into saturated fats by adding hydrogen. It’s produces saturated fats and unsaturated fats with trans double bonds (which contribute to cardiovascular diseases more than saturated fats).
74
New cards
What are the functions of fat?
Energy storage (in adipose cells) (more than twice as much as polysaccharides) - cushioning of vital organs - insulating the body.
75
New cards
What is the structure of phospholipids?
A glycerol with two fatty acids and a negatively charged phosphate group which can be linked to other small molecules.
76
New cards
How do phospholipids react in water?
They self assemble into a bilayer with hydrophobic portions (tails: fatty acids)on the inside and hydrophilic portion (heads: glycerol and phosphate group) on outside.
77
New cards
Where does phospholipid diversity come from?
From the differences in the fatty acids and in the groups attached to the phosphate group.
78
New cards
What is the structure of steroids?
It’s carbon skeleton consists of four fused rings.
79
New cards
What is cholesterol?
It is the antecedent of other steroids, it is synthesized in the liver of vertebrates.
80
New cards
What is a catalyst?
An enzymatic proteins that speed up chemical reactions without being consumed.
81
New cards
Which molecules are the most complexly structured?
Proteins
82
New cards
What are polypeptides?
Polypeptides are polymers of amino acids.
83
New cards
What is a protein?
It is one or more polypeptides.
84
New cards
What are the functions of proteins?
Enzymatic - defensive - storage – transport - hormonal – receptor - contractile and motor - structural.
85
New cards
What is an amino acid?
A polypeptide monomer that consists of an asymmetric carbon (alpha carbon) attached to a hydrogen, carboxyl group(ionized in cell pH), amino group, and a side chain R group.
86
New cards
What is a peptide bond?
It is the covalent bond that holds the carboxyl group of one amino acid to the amino group of another amino acid in a polypeptide.
87
New cards
What is the polypeptide backbone made of?
Carboxyl group, amino group, carbon, hydrogen. (Fixed)
88
New cards
What determines the chemical nature of a protein molecule?
The kind and sequence of sides chains because they greatly outnumbered the terminal groups.
89
New cards
What does a polypeptide end with?
A carboxyl end (C-terminus) and an amino end (N-terminus)
90
New cards
What is the primary structure of a protein?
A linked sequence of amino acids that is determined by inherited genetic information. It dictates secondary and tertiary structures.
91
New cards
What is secondary structure of a protein?
The coils and folds of amino acid sequence as a result of hydrogen bonds between the repeating constituents of the polypeptide backbone.
92
New cards
What are two types of secondary structure?
Alpha helix: A coil held together by hydrogen bonds between every fourth amino acid.
Beta pleated sheet: beta strands of the polypeptide chain lying parallel to each other and connected by hydrogen bonds between parts of the backbones.
Beta pleated sheet: beta strands of the polypeptide chain lying parallel to each other and connected by hydrogen bonds between parts of the backbones.
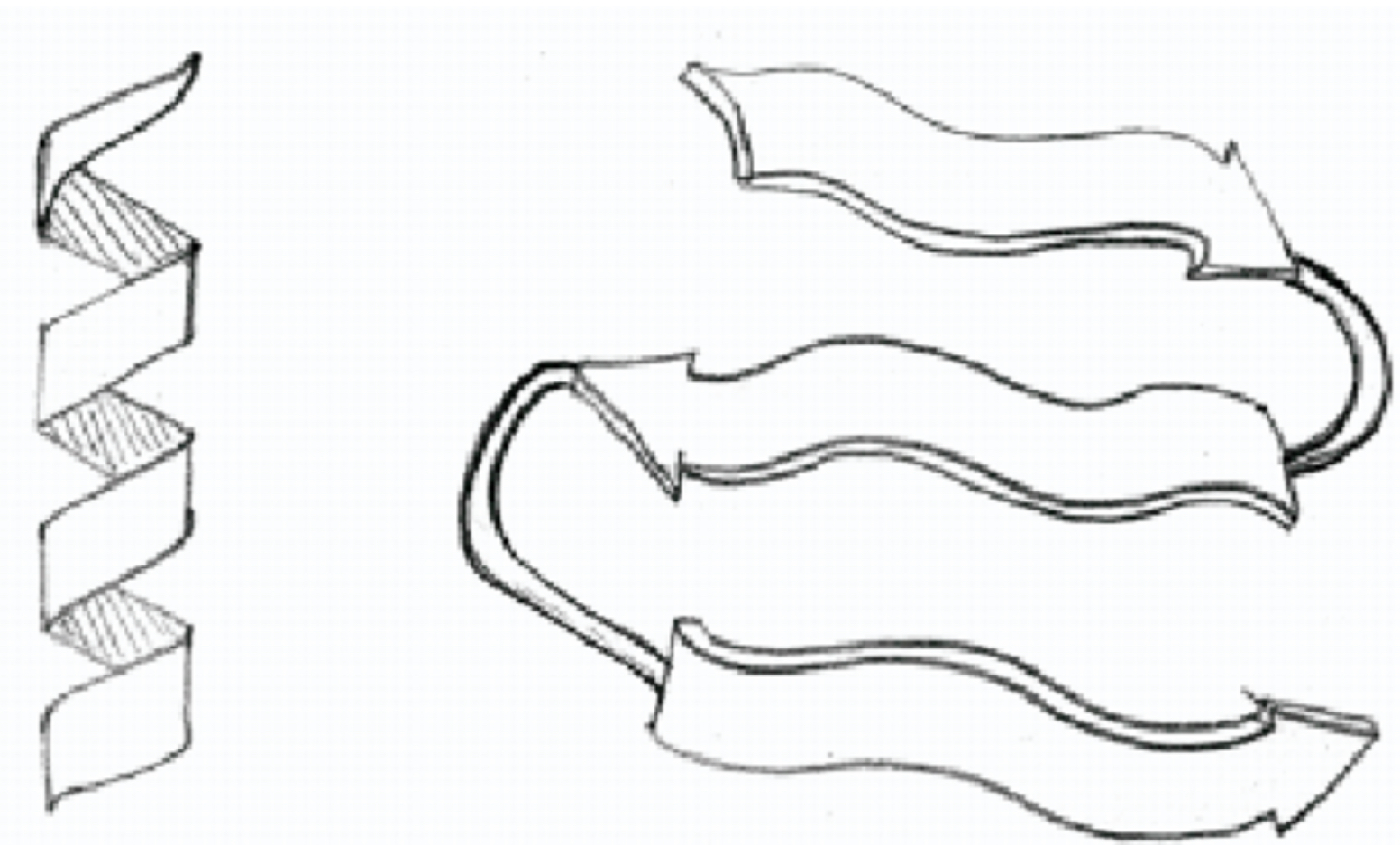
93
New cards
What is a tertiary structure of a protein?
The overall 3D shape of the polypeptide resulting from interactions of sides chains or R groups of amino acids.
94
New cards
What is a hydrophobic interaction?
It is an interaction caused by the exclusion of nonpolar substances by water molecules, where non polar substances are held together by the Vanderwall forces and hydrogen bonds hold the polar parts together.
95
New cards
What is a disulfide bridge?
It happens when the sulfur from one cysteine bonds to the sulfur of another cysteine and rivers the protein together.
96
New cards
What is a quaternary structure?
It is the proteins structure that results from more than one polypeptide chain bonded together such as a hemoglobin.
97
New cards
What are the key factors that determine proteins structure?
The amino acid sequence, the physical and chemical conditions of the proteins environment.
98
New cards
What is denaturation?
It is when a protein unravels and loses its native shape due to environmental changes. When altering agents are removed a protein chain gets back to its shape.
99
New cards
What are chaperonins or chaperon proteins?
Protein molecules that assist to the folding of other proteins by protecting them from bad influences in the cytoplasmic environment.
100
New cards
What determines the primary structure of a protein?
The amino acid sequence is programmed by a gene which is made of DNA.