General Chemistry
5.0(1)
Card Sorting
1/119
Last updated 4:35 PM on 10/27/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
120 Terms
1
New cards
Matter
– anything that has mass and occupies space.
2
New cards
Matter is made up of small particles
The particles have spaces between them:
1. Inter-Particle Spaces
2. Inter-Molecular Spaces
Particles attract each other
1. Intermolecular Forces
2. Intramolecular Forces
Particles of matter are in constant motion
The particles have spaces between them:
1. Inter-Particle Spaces
2. Inter-Molecular Spaces
Particles attract each other
1. Intermolecular Forces
2. Intramolecular Forces
Particles of matter are in constant motion
Kinetic Molecular Theory of Matter
3
New cards
Intermolecular Forces
these are attractive forces between atoms/molecules, and these become stronger as the particles move closer together.
4
New cards
Intramolecular Forces
are forces that holds atoms together in a molecule, also known as bonds.
5
New cards
Solid
- Definite shape
- Constant Volume
- Nearly incompressible
- Particles are very close to each other
- Particles vibrates in fixed position relative to one another
- Constant Volume
- Nearly incompressible
- Particles are very close to each other
- Particles vibrates in fixed position relative to one another
6
New cards
Intermolecular Spaces of solids are very small. Intermolecular Forces are stronger
Kinetic Molecular Model of Solid:
7
New cards
Intermolecular Spaces of liquids larger than solids. Intermolecular Forces is less /weaker than solids.
Kinetic Molecular Model of Liquid:
8
New cards
Liquid
- No definite shape. It takes the shape of the container.
- Constant Volume.
- Slightly compressible
- Particles are neither too close nor too far from each other.
- Particles moves freely among themselves, but clump together.
- Constant Volume.
- Slightly compressible
- Particles are neither too close nor too far from each other.
- Particles moves freely among themselves, but clump together.
9
New cards
Gas
- No definite shape. It takes the shape of the container.
- No definite volume
- It can be expanded or compressed
- Particles are far apart
- Particles are independent of one another.
- Particles move in random motion
- No definite volume
- It can be expanded or compressed
- Particles are far apart
- Particles are independent of one another.
- Particles move in random motion
10
New cards
Intermolecular Spaces of Gases are very large. Intermolecular Forces are very weak.
Kinetic Molecular Model of Gas:
11
New cards
Chemical Bonds
– force of attraction that holds two atoms together in a molecular or ion pair.
12
New cards
Octet Rule
– bonds are formed either by transferring or sharing of electrons to achieve a stable configuration. Elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas.
13
New cards
Ionic Bonds and Covalent Bonds
Types of Chemical Bond:
14
New cards
Ionic Bonds
– formed between metal and nonmetal elements. When Metals lose electron, it becomes positively-charged (Cation) while nonmetals become negatively-charged (Anion) when it accepts electrons. Ionic bonds involve in the attraction between the two charged Ions.
15
New cards
Cation
positively-charged ion
16
New cards
Anion
negatively-charged ion
17
New cards
Ionic Bonds
This bond holds the ions together in an Ionic compound.
18
New cards
Covalent Bonds
also called molecular bond
19
New cards
Covalent Bonds
It is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. Formed between two nonmetal atoms with identical or relatively close electronegativity values.
20
New cards
Single Covalent Bond
─ two atoms share one pair of electrons.
21
New cards
Double Covalent Bond
─ two atoms share two pairs of electrons.
22
New cards
Triple Covalent Bond
─ two atoms share three pairs of electrons.
23
New cards
Molecular Compound
– the compound that consists of atoms bonded together by shared electrons.
24
New cards
Diatomic Gases
─ Made up of atoms of the same elements (Ex. F2, O2, N2).
25
New cards
Binary Acids
─ Hydrogen, a nonmetal element, shares electron with Halogens to form binary acids and to achieve stability. (Ex. HF, HCl, HBr)
26
New cards
1. Diatomic Gases
2. Binary Acids
2. Binary Acids
Examples of Molecular Compounds:
27
New cards
Bond Polarity
– electron sharing in covalent bonds may be equal or not between two atoms.
28
New cards
Polar & Nonpolar
2 kinds of covalent bonds
29
New cards
Nonpolar Covalent Bond
– electrons are equally shared between atoms. This means their electronegativities are almost the same. (Ex. H2, F2, Cl2, Br2 O2, & N2 ─ Diatomic gases)
30
New cards
Polar Covalent Bond
– electrons are shared unequally, resulting in one atom being partially negative (δ-) and the other being partially positive (δ+). (Ex. HF)
31
New cards
Electronegativity
– the tendency for an atom to attract electrons to itself when it is chemically combined with another element.
32
New cards
F (4.0)
O (3.5)
N (3.0)
Cl (3.0)
O (3.5)
N (3.0)
Cl (3.0)
Four most electronegative elements:
33
New cards
Electronegativity Difference
– provides an estimate to determines the type of chemical bond the most likely to exists in a compound.
34
New cards
Non-polar Covalent
─ bond formed when difference in electronegativity is 0.
35
New cards
Polar Covalent Bond
─ formed when electronegativity difference is less than 1.5 but not equal to 0.
36
New cards
Ionic Bond
─ formed when difference in the electronegativity is equal to or greater than 1.5
37
New cards
Dipole Moments
occur when there is a separation of charge. They can occur between two ions in an ionic bond or between atoms in a covalent bond; arise from differences in electronegativity. Arrow points toward the more e-neg atom.
38
New cards
Dipole Moments
measure of the polarity of the molecule, direction of the polar bond in a molecule.
39
New cards
Dipolar Molecule or Dipole
– a molecule that has 2 poles (charged regions), like H-Cl.
40
New cards
Nonpolar Molecules
– Dipole moments are symmetrical and cancel out.
41
New cards
Polar Molecules
– Dipole moments are asymmetrical and don’t cancel. Therefore, they have asymmetrical shape (lone pairs) or asymmetrical atoms.
42
New cards
Geometry
– arrangement of atoms in a molecule.
43
New cards
Intramolecular Forces
– are the forces that hold atoms together within a molecule.
44
New cards
Inter-Ionic Forces
– are forces that hold ions together.
45
New cards
Intermolecular Forces
– are forces that exist between molecules.
– this force holds the molecules together which can be attractive or repulsive. – – these attractive forces are much weaker than bonding forces.
– determines the physical properties of molecules like their boiling point, melting point, density, and enthalpies of fusion and vaporization.
– are accountable for the properties of substances.
– explain why substances exist as solids, liquids, or gases at room temperature.
– this force holds the molecules together which can be attractive or repulsive. – – these attractive forces are much weaker than bonding forces.
– determines the physical properties of molecules like their boiling point, melting point, density, and enthalpies of fusion and vaporization.
– are accountable for the properties of substances.
– explain why substances exist as solids, liquids, or gases at room temperature.
46
New cards
1. Dipole-Dipole
2. Ion-Dipole
3. Dispersion / London Forces
4. Hydrogen Bond
2. Ion-Dipole
3. Dispersion / London Forces
4. Hydrogen Bond
Four Types of Intermolecular Forces (involving covalent molecules)
47
New cards
1. Dipole-Dipole
2. Ion-Dipole
3. Dispersion / London Forces
2. Ion-Dipole
3. Dispersion / London Forces
Van Der Waals Forces
48
New cards
Johannes Diderik Van Der Waals
– was born on November 23, 1837 in Leyden, The Netherlands.
– in his 1873 thesis, Van Der Waals noted the non-ideality of real gases and attributed it to the existence of intermolecular interactions.
– in his 1873 thesis, Van Der Waals noted the non-ideality of real gases and attributed it to the existence of intermolecular interactions.
49
New cards
Dipole-Dipole Forces
– attractive forces existing between polar molecules (molecules that exhibit dipole moment), such as HCl.
50
New cards
Dipole-Dipole Forces
These forces occur when the partially positively charged part of a molecule interacts with the partially negatively charged part of the neighboring molecule. All polar molecules have a partial negative end and partial positive end which are attracted to each other.
51
New cards
Dipole
– the existence of partial positive and partial negative poles because there is unequal sharing of electron between H and Cl atoms.
52
New cards
Dipole-Dipole Forces
This force is weaker than ionic and hydrogen or covalent bonds.
53
New cards
Ion-Dipole Forces
exist in the attraction between a charged particle called ion (which can be a positively charged cation or a negatively charged anion) and a polar (dipole molecule).
54
New cards
Ion-Dipole Interaction
An ion is a charged atom because it has gained or lost one or more electrons. It can be either positively charged cation or negatively charged anion. The partial charges at the ends of the dipole molecules make an ______________.
55
New cards
Dispersion or London Forces
– are the weakest attractive force that are formed due to the temporary dipoles induced in non-polar molecules.
56
New cards
Dispersion or London Forces
This force is also called induced-dipole attraction.
57
New cards
Induced Dipole
distortion will result in temporary dipoles in the nonpolar molecule which is called ________.
58
New cards
Induced Dipole
– is the separation of the positive and negative charges in a nonpolar molecule due to its nearness of an ion or polar molecule.
59
New cards
Dispersion or London Forces
- exists when the electrons in two adjacent atoms attract and induce temporary dipoles.
60
New cards
Dispersion or London Forces
- exist between all types of molecules, whether ionic or covalent —polar or nonpolar.
61
New cards
Induced Dipole Force
– forces between nonpolar where electrons are evenly distributed; no dipole.
62
New cards
Induced Dipole-Induced Dipole Force
molecules that have induced dipoles may also induce neighboring molecules to have dipole moments, so a large network of induced dipole-induced dipole interactions may exist.
63
New cards
Ion-Induced Dipole Interaction
– when the induced dipole is due to the interaction between an ion and non-polar molecule.
64
New cards
Dipole-Induced Dipole Interaction
– when the induced dipole is due to the interaction between a polar and nonpolar molecule.
65
New cards
Hydrogen Bond
– is a special kind of dipole-dipole interaction that occurs specifically between a hydrogen (H) atom bonded to either an Oxygen, Nitrogen, or Fluorine atom (highly electronegative atoms, F, O, and N).
66
New cards
Hydrogen Bond
partially positive end of hydrogen is attracted to the partially negative end of the oxygen, nitrogen, or fluorine of another molecule.
67
New cards
Hydrogen Bonding
is a relatively strong force of attraction between molecules, and considerable energy is required to break hydrogen bonds.
68
New cards
Hydrogen bonding
plays an important role in biology; for example, hydrogen bonds are responsible for holding nucleotide bases together in DNA and RNA.
69
New cards
Surface Tension
– could be defined as the property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of the water molecules.
– it is the measure of the elastic force in the surface of a liquid.
– it is the amount of energy required to stretch or increase the surface of a liquid by a unit area.
– it is manifested as some sort of skin on the surface of a liquid or in a drop of liquid.
– it is the measure of the elastic force in the surface of a liquid.
– it is the amount of energy required to stretch or increase the surface of a liquid by a unit area.
– it is manifested as some sort of skin on the surface of a liquid or in a drop of liquid.
70
New cards
Manifestations of Surface Tension:
- Formation of a meniscus
- Capillary action which results from a combination of:
1. Cohesion
2. Adhesion
- Capillary action which results from a combination of:
1. Cohesion
2. Adhesion
71
New cards
Capillary Action
– it is the tendency of a liquid to rise in narrow tubes or be drawn into small openings such as those between grains of a rock.
– also known as capillarity, is a result of intermolecular attraction between the liquid and solid materials.
– also known as capillarity, is a result of intermolecular attraction between the liquid and solid materials.
72
New cards
Capillary Action
– straw lowered into water
73
New cards
Cohesion
– is the attraction between like molecules, cohesive forces.
74
New cards
Adhesion
– is an attraction between unlike molecules (such as those in water and in the particles that make up the glass tube), adhesive forces
– these forces also define the shape of the surface of a liquid in a cylindrical container (the meniscus!)
– these forces also define the shape of the surface of a liquid in a cylindrical container (the meniscus!)
75
New cards
Adhesion, because they stick to the container, not with each other.
Water is having?
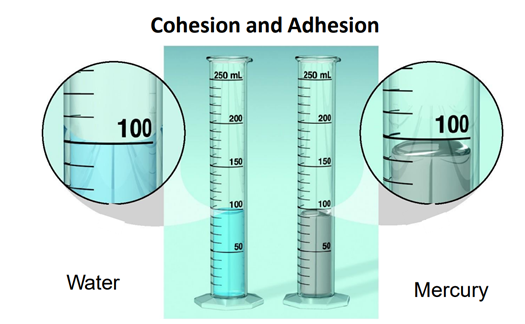
76
New cards
Cohesion, because they stick with each other, not in the container.
Mercury is having?
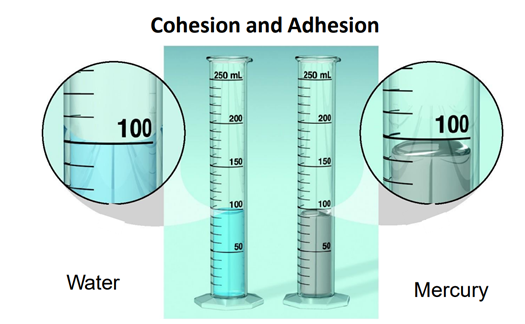
77
New cards
Viscosity
– a measure of a fluid’s resistance to flow.
– units: N.s/m2
– the higher the viscosity the greater the resistance to flow.
– varies inversely with temperature
– stronger intermolecular forces produce higher viscosities.
– units: N.s/m2
– the higher the viscosity the greater the resistance to flow.
– varies inversely with temperature
– stronger intermolecular forces produce higher viscosities.
78
New cards
Glycerol
– high viscosity due to:
• three hydrogen bonding sites
• molecular shape
• three hydrogen bonding sites
• molecular shape
79
New cards
Vapor Pressure
– the pressure exerted by a vapor in equilibrium with its liquid state in a closed container.
– liquid molecules at the surface escape into the gas phase.
– these gas particles create pressure above the liquid in a closed container.
– liquid molecules at the surface escape into the gas phase.
– these gas particles create pressure above the liquid in a closed container.
80
New cards
Closed System (constant temperature)
As liquid continues to vaporize, a point is reached: for every molecule that vaporizes, one condenses.
81
New cards
Equilibrium
When Vaporization = Condensation we have reached ___________
82
New cards
Vapor Pressure
– the pressure of a gas at equilibrium with the liquid in a closed system.
– is a characteristic property
– is a characteristic property
83
New cards
1. Sealed container – Closed system
2. Constant condition ([ ], T, P, V)
2. Constant condition ([ ], T, P, V)
Vapor Pressure Equilibrium will only be reached with:
84
New cards
• Compares vapor pressure to normal atmospheric pressure.
• Measures difference in height on both sides
• Measures difference in height on both sides
Vapor Pressure can be measured by a manometer:
85
New cards
Pvap = Patm – h
(vapour pressure < air pressure)
86
New cards
Pvap = Patm + h
(vapour pressure > air pressure)
87
New cards
• Intermolecular forces
• Temperature
• Pressure
• Temperature
• Pressure
Vapor Pressure is influenced by:
88
New cards
Intermolecular forces
– stronger attraction restricts phase change
89
New cards
→ less energy needed to break IMF → easier for all particles to phase change (regardless of temperature) → increasing rate of vaporization → increasing vapor pressure.
weaker Intermolecular Forces (vapor pressure)
90
New cards
→ more energy needed to break IMF → harder for all particles to phase change (regardless of temperature) → decreasing rate of vaporization → decreasing vapor pressure.
stronger Intermolecular Forces (vapor pressure)
91
New cards
Volatile (Volatility)
– compounds that easily vaporize (have low IMF – Intermolecular forces).
92
New cards
Temperature
– energy is required to phase change.
93
New cards
→ increases the kinetic energy of all particles → increasing the number of particles with the energy to break IMF → increasing rate of vaporization → increasing vapor pressure.
increasing temperature (vapor pressure)
94
New cards
→ decreases the kinetic energy of all particles → decreasing the number of particles with the energy to break IMF → decreasing rate of vaporization → decreasing vapor pressure.
cooling/decreasing temperature (vapor pressure)
95
New cards
True
Vapor Pressure increases with increasing temperature
96
New cards
as the liquid gains kinetic energy, the molecules can overcome the intermolecular forces of attraction
As temperature increases, the amount of vapor generated by a liquid in a closed container increase. This occurs because ___________________ that are prevalent in the liquid phase.
97
New cards
Pressure
forces particles into liquid state
98
New cards
→ added force restricting particle motion → harder for all particles to phase change (regardless of temperature) → decreasing rate of vaporization → decreasing vapor pressure
increasing pressure (vapor pressure)
99
New cards
an open system must contend with atmospheric pressure
– air molecules colliding and pushing on the surface of the liquid making vaporization more difficult.
100
New cards
Evaporation
– transition from a liquid to a gas below a substances boiling point.
– occurs when molecules at the liquids surface are moving fast enough to escape into the gas phase.
– also called vaporization.
– requires energy to overcome intermolecular forces between the molecules of the liquid.
– some of the liquid particles have enough kinetic energy to overcome the forces of attraction around them and escape into the gas phase.
– high energy molecules escape the surface.
– occurs when molecules at the liquids surface are moving fast enough to escape into the gas phase.
– also called vaporization.
– requires energy to overcome intermolecular forces between the molecules of the liquid.
– some of the liquid particles have enough kinetic energy to overcome the forces of attraction around them and escape into the gas phase.
– high energy molecules escape the surface.