Thermodynamic Work
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
dx
(1)
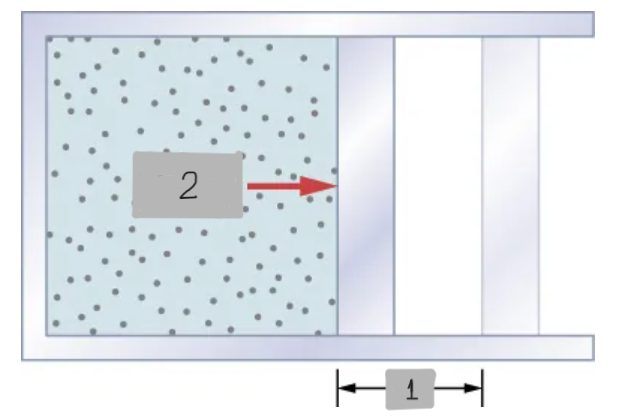
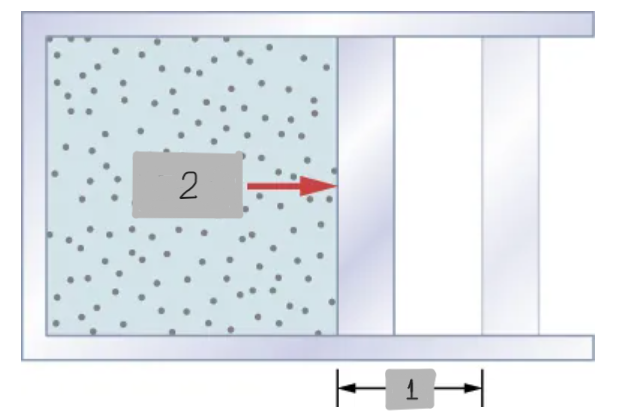
F = pA
(2)
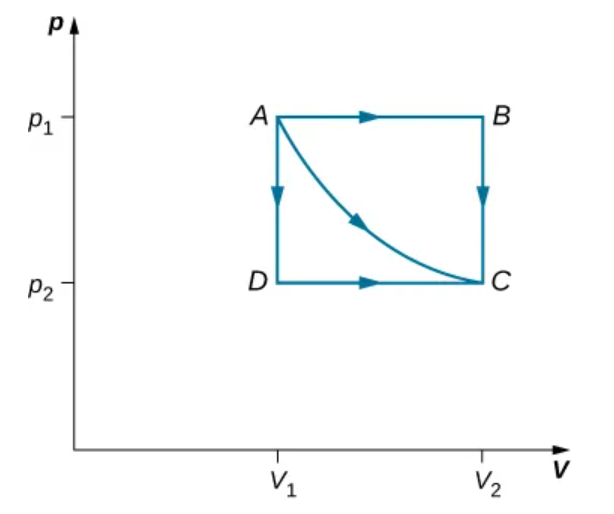
The paths ABC, AC, and ADC represent three different quasi-static transitions between the equilibrium states A and C.
Thermodynamic Work
The work done when a system’s boundary moves under a force, like how, a gas expands or compresses a piston.
dW = pdV
Work done by a Gas (Infinitesimal) represented as Work done by a system during a tiny change in Volume at pressure p
W = ∫(from V1 to V2) pdV
Work Done Over a Finite Volume Change
Positive Work
Gas expands (V2 > V1)
Negative Work
Gas is compressed (V2 < V1)
Quasi-Static Work
A process carried out slowly enough that the system remains in thermal equilibrium at all times, allowing a well-defined p(V) relationship.
Path Dependance of Work
Work is not a state function
Different thermodynamic paths between the same two states yield different work values
W = nRTln(V2/V1)
Isothermal Work (Ideal Gas)
Only valid when the temperature remains constant
Isobaric Work
Work done during a constant-pressure process.
W = p(V2 - V1)
Mathematical representation of the Isobaric Work
Internal Energy (Eint)
The sum of all microscopic kinetic and potential energies of all particles in the system.
Components of Molecular Kinetic Energy
Includes translational, rotational, and vibrational kinetic energy of molecules.
Components of Molecular Potential Energy
Associated with intermolecular interactions (negligible for an ideal gas)
Eint = (3/2)nRT
Internal Energy of an Ideal Monoatomic Gas. (Depends only on the Temperature)
K̅ = (3/2)((k_B)T)
Average Translational Kinetic Energy per Molecule
translational kinetic energy
In an ideal monoatomic gas, only __________________________ contributes to the internal energy, rotational, vibrational and intermolecular potential energies are absent.
redistribute, energy, collisions
Molecules ____________ ______ through __________ until both gases reach the same temperature (thermal equilibrium)
Yes it is true
Is it true that for an ideal monoatomic gas, temperature directly determines average kinetic energy and therefore internal energy.
No Effect of Bulk Motion on Internal Energy
Internal energy does not depend on system’s location or macroscopic motion (e.g., moving the gas to a higher floor does not changes the Eint).
Work By The Gas
Expansion → Gas pushes surroundings.
Work On The Gas
Compression → Surroundings push the gas
pV diagram
A plot of pressure v/s volume used to visualize processes
Area under the curve equals the work done by the gas.
Isothermal Process
Thermodynamic process during which temperature remains constant
Internal energy stays constant for ideal gases.