Ella Kulman ICS
1/192
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
193 Terms
define inflammation
a local physiological response to tissue injury
what is an advantage of inflammation?
inflammation can destroy invading microorganisms and can prevent spread of infection
what is a disadvantage of inflammation?
can produce disease and lead to distorted tissues with permanently altered function
4 outcomes of inflammation
1. resolution
2. suppuration
3. organisation (scar tissue formation)
4. progression onto chronic inflammation
give 6 causes of acute inflammation
1. microbial infections (bacteria/viruses)
2. chemicals
3. physical agents (trauma/burns/frostbite)
4. hypersensitivity reactions (TB)
5. bacterial toxins
6. tissue necrosis
what does viral infection result in?
cell death due to intracellular multiplication
what does bacterial infection result in?
release of exotoxins (involved in the initiation of inflammation) or endotoxins
5 cardinal signs of inflammation
1. redness (rubor)
2. swelling (tumor)
3. pain (dolor)
4. heat (calor)
5. loss of function
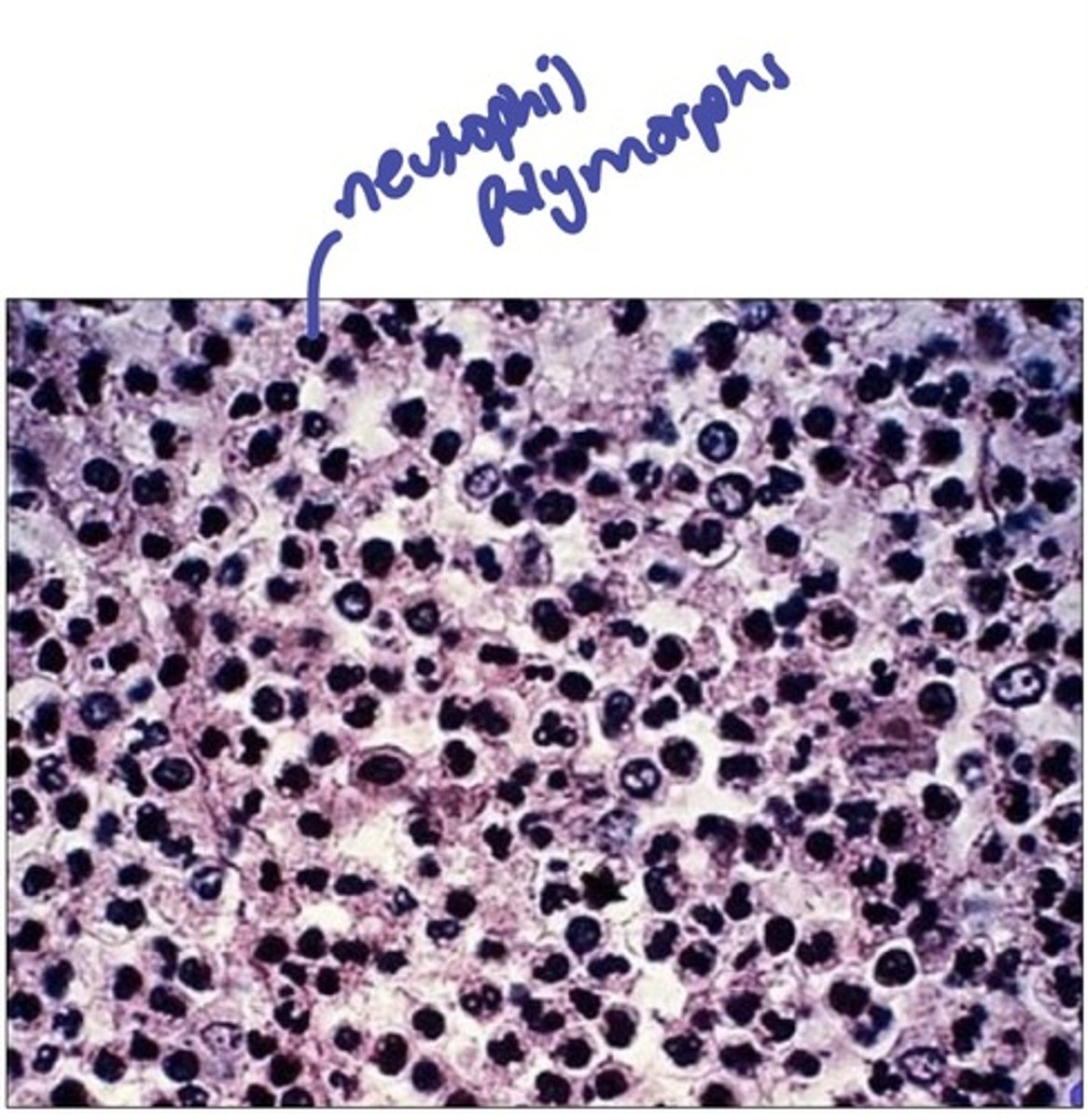
how can acute inflammation be diagnosed histologically?
by looking for the presence of neutrophil polymorphs
give 3 endogenous chemical mediators of acute inflammation
1. bradykinin
2. histamine
3. nitric oxide
what are 4 systemic effects of acute inflammation?
1. fever
2. feeling unwell
3. weight loss
4. reactive hyperplasia of the reticuloendothelial system
what cells are involved in chronic inflamation?
macrophages and plasma cells (B and T lymphocytes)
what cell can form when several macrophages try to ingest the same particle?
multinucleate giant cell
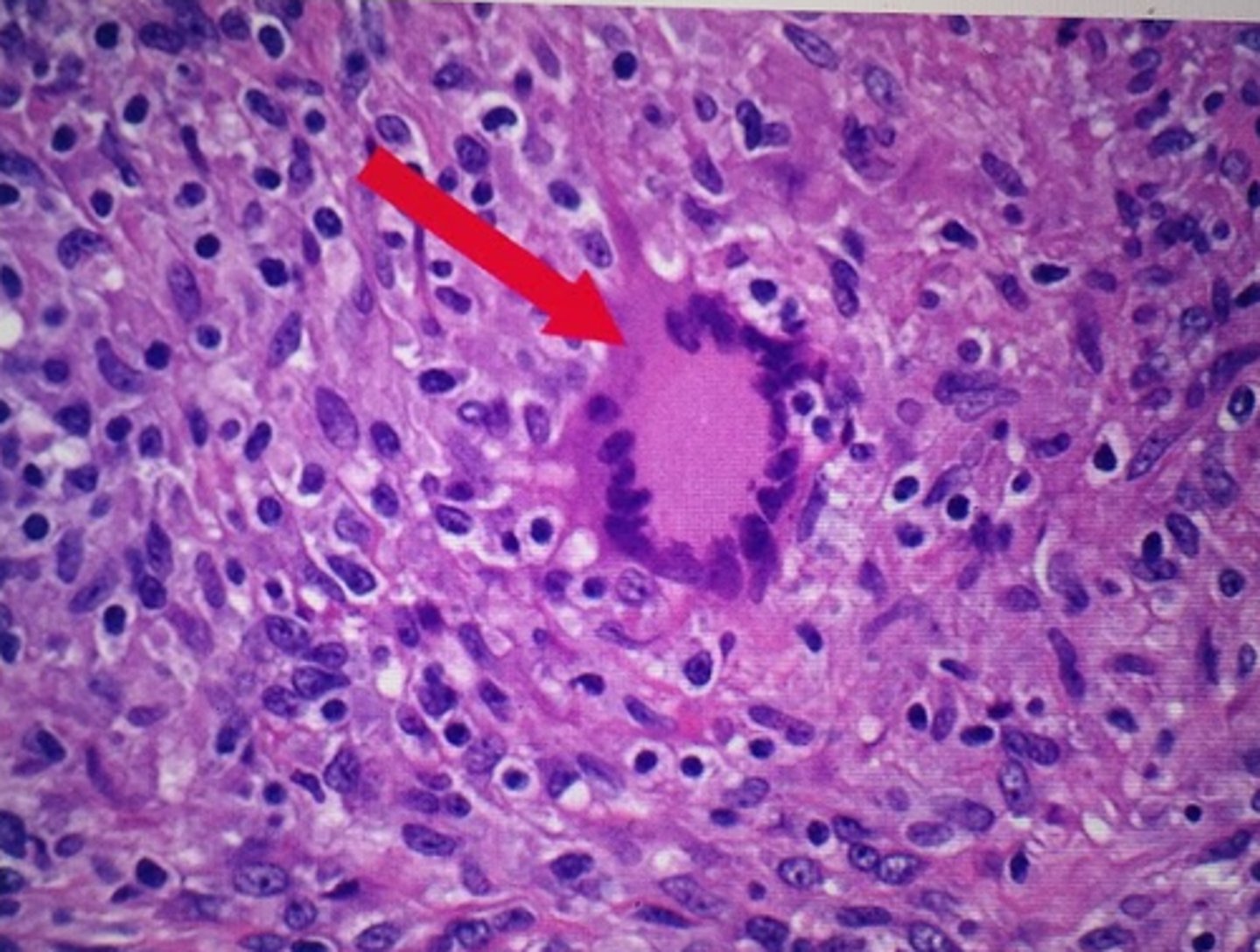
give 4 causes of chronic inflammation
1. primary chronic inflammation
2. transplant rejection
3. recurrent acute inflammation
4. progression from acute inflammation
give examples of primary chronic inflammation
1. infective substances having resistance to phagocytosis (eg TB/leprosy)
2. endogenous materials (eg uric acid crystals)
3. exogenous materials (eg asbestos)
4. autoimmune diseases (eg chronic gastritis, RA etc)
5. other chronic inflammatory diseases (eg chronic inflammatory bowel disease)
in which type of inflammation would you see neutrophil polymorphs?
acute inflammation
what are some macroscopic features of chronic inflammation
1. chronic ulcer
2. chronic abscess cavity
3. granulomatous
4. fibrosis
what is granulation tissue?
composed of small blood vessels in a connective matrix with myofibroblasts (important in healing and repair)
define granuloma
an aggregate of epithelioid histocytes
give an example of a granulomatous disease
TB, leprosy, crohn's disease and sarcoidosis
the activity of what enzyme in the blood can act as a marker for granulomatous disease
angiotensin converting enzyme
what is the difference between resolution and repair?
resolution is when the initiating factor is removed and the tissue is able to regenerate, in repair the initiating factor is still present and the tissue is unable to regenerate
5 types of cells capable of regeneration
1. hepatocytes
2. osteocytes
3. pneumocytes
4. blood cells
5. gut and skin epithelial
name 2 cells incapable of regeneration
1. myocardial cells
2. neuronal cells
define an abcess
acute inflammation with a fibrotic wall
define thrombosis
formation of a solid mass from blood constituents in an intact vessel in the living
give 2 reasons why thrombosis formation is uncommon
1. laminar flow
2. non sticky endothelial cells
what are the 3 factors that can lead to thrombosis formation?
1. change in vessel wall
2. change in blood constituents
3. change in blood flow
define embolus
a mass of material (often a thrombus) in the vascular system that is able to become lodged in a vessel and block it
define ischaemia
decreased blood flow
define infarction
decreased flow with subsequent cell death
why are tissues with an end arterial supply more susceptible to infarction?
they only have a single arterial supply and so if this is interrupted then infarction is more likely
give 3 examples of organs with a dual arterial supply
1. lungs (bronchial arteries and pulmonary veins)
2. liver (hepatic arteries and portal veins)
3. some areas of the brain around the circle of willis
what complication can occur if ischaemia is rectified?
re-perfusion injury can occur due to the release of waste products
what are the consequences of an arterial embolus?
it can go anywhere - so could be stroke, MI, gangrene etc
what are the consequences of a venous embolus?
it will reach the vena cava then the pulmonary arteries and become lodged in the lungs, causing a pulmonary embolism (decreased lung perfusion)
through which blood system does an embolus have to travel to result in a pulmonary embolism?
venous system
what common drug can be used to prevent thrombosis?
aspirin
define atherosclerosis
inflammatory process characterised by hardened plaques in the intima of a vessel wall

is atherosclerosis more common in the systemic or pulmonary circulation?
systemic because its higher pressure (endothelial damage theory)
what are the 3 main components of an atheromatous plaque?
1. lipids
2. fibrous tissue
3. lymphocytes
give 5 risk factors for atherosclerosis
1. smoking
2. hypertension
3. hyperlipidaemia
4. uncontrolled diabetes mellitus
5. lower socioeconomic status
what can be done to prevent atherosclerosis?
reduce risk factors and take low dose aspirin regularly
what theory is considered the primary cause of atherosclerosis?
endothelial cell damage
why can cigarette smoking lead to atherosclerosis?
releases free radicals, nicotine and CO into the body which all damage endothelial cells
why can hypertension lead to atherosclerosis?
high BP = greater force exerted on the endothelial cells which can lead to damage
define apoptosis
programmed cell death of a single cell
what is the role of p53 protein?
it looks for DNA damage, if damage is present p53 switches on apoptosis
what protein can switch on apoptosis if DNA damage is present?
p53 protein
give an example of a disease where there is a lack of apoptosis and why
cancer - mutations in p53 mean cell damage isn't detected
give an example of a disease where there is too much apoptosis
HIV
define necrosis
unprogrammed death of a large number of cells due to an adverse event
give 3 examples of events that can lead to necrosis
1. frost bite
2. avascular necrosis
3. infarction
define hypertrophy
increase in size of a tissue due to an increase in the size of its constituent cells
define hyperplasia
increase in the size of a tissue due to an increase in the number of its constituent cells
define atrophy
decrease in tissue size due to either decreased cell size or number of cells
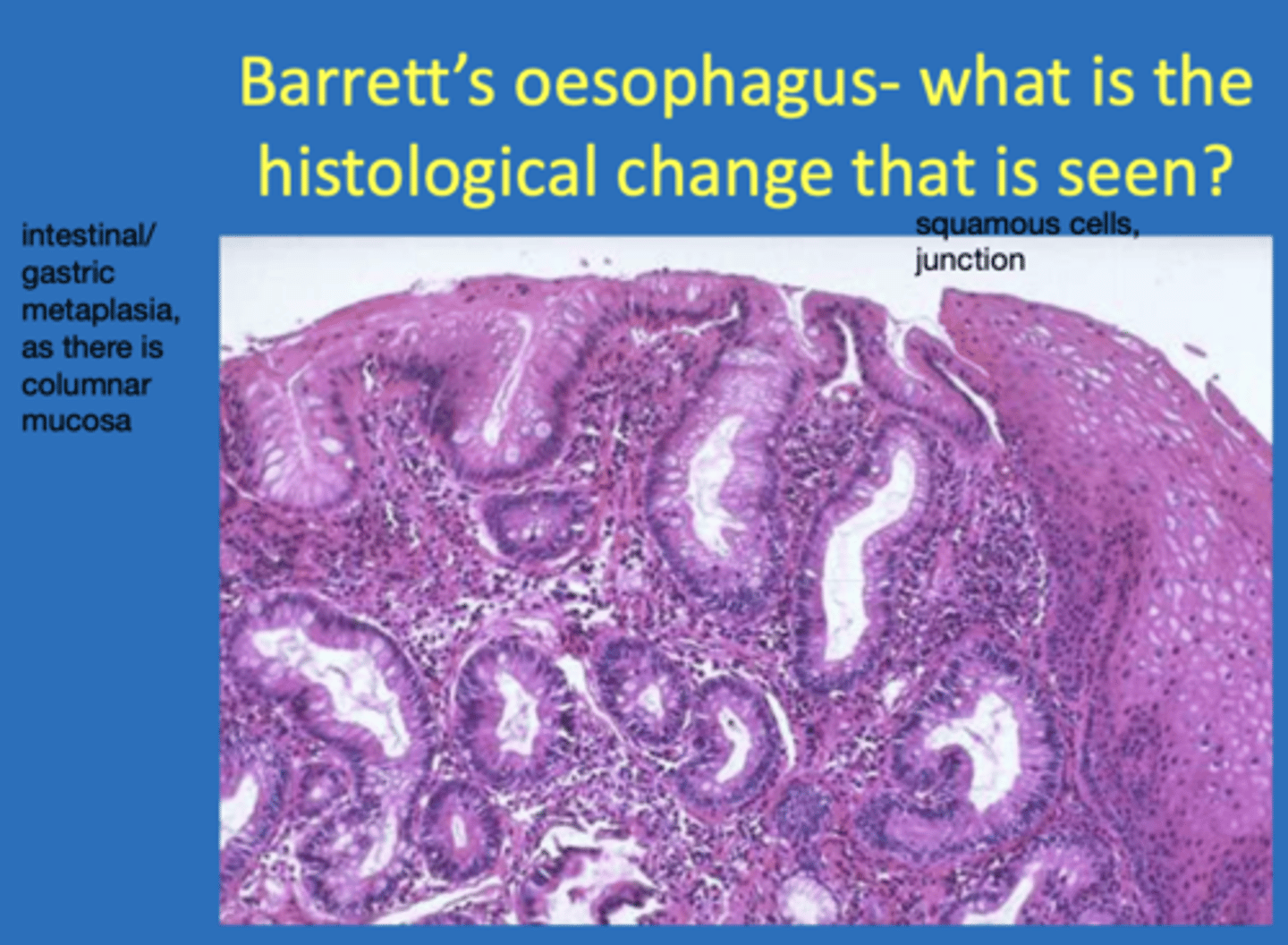
define metaplasia
a change in differentiation of a cell from one fully differentiated type to another (eg fibrous tissue to bone or mucosal epithelia changing)
give an example of a disease that demonstrates metaplasia
barrett's oesophagus - the cells at the lower end change from stratified squamus to columnar
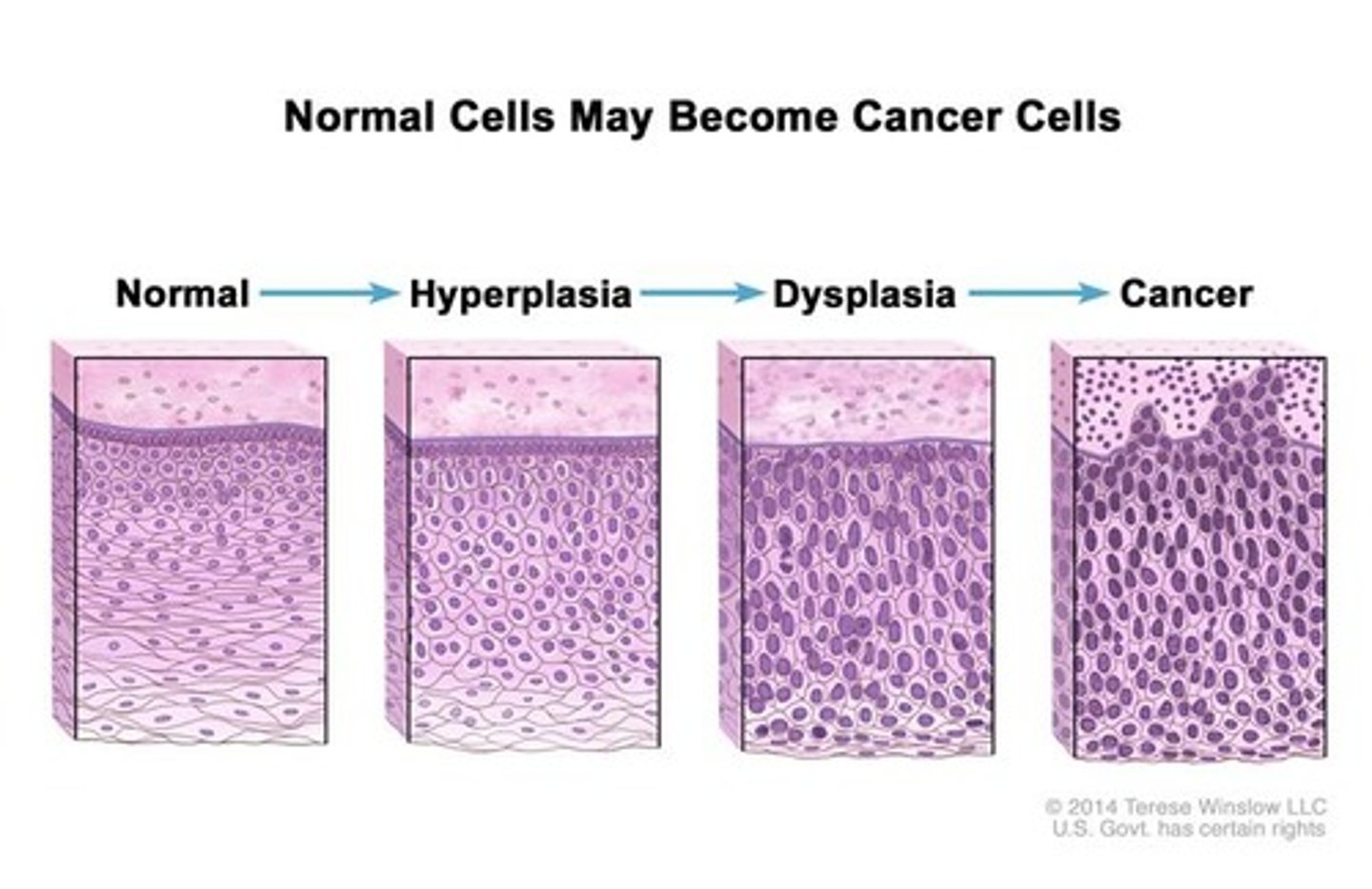
define dysplasia
morphological changes seen in cells in the progression to becoming cancer (cells become more 'jumbled up')
why can excision be a cure for basal cell carcinoma?
because BCC doesn't metastasise
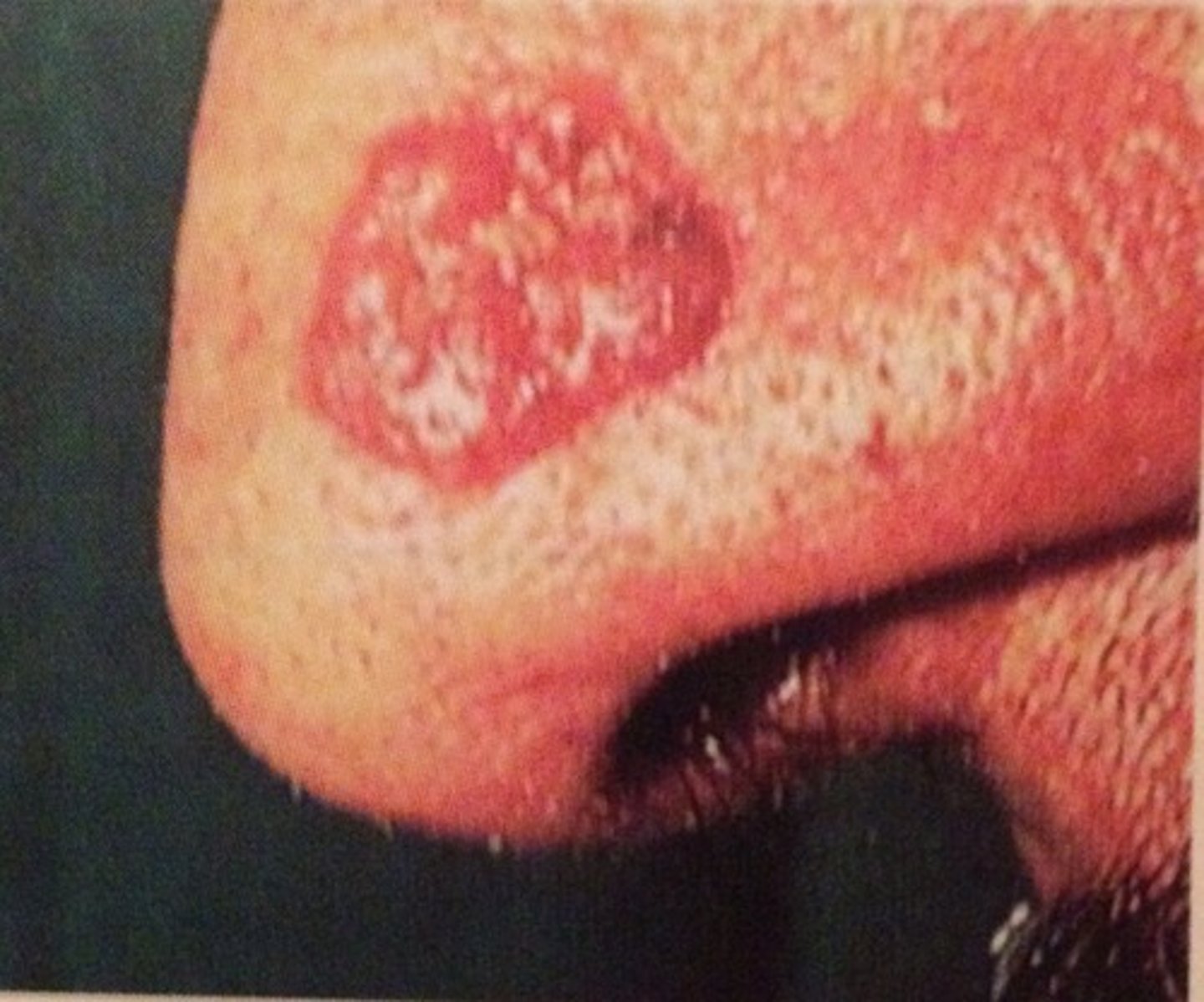
what is the standard treatment for leukaemia and why?
chemo - leukaemia is systemic so can't be excised
give an example of 5 carcinomas that can spread to bone
1. breast
2. kidney
3. lung
4. prostate
5. thyroid
give an example of a carcinoma that can spread to the axillary lymph nodes
breast carcinomas
why is adjuvant therapy (used after primary treatment like surgery to prevent reoccurence) often used to treat carcinomas?
micrometastes are possible even if a tumour is excised and so adjuvant therapy is given to suppress secondary tumour formation
for what kind of carcinomas would targeted chemo be most effective against?
slower dividing tumours, like lung/colon/breast
what kind of drugs can be used in targeted chemo?
monoclonal antibodies (MAB) and small molecular inhibitors (SMI)
what 3 mechanisms do tumour cells use to evade host immune defence in the blood?
1. platelet aggregation
2. adhesion to other tumour cells
3. they shed surface antigens so as to 'distract' lymphocytes
give an example of a malignant tumour that often spreads to the lung
sarcoma (via vena cava → heart → pulmonary arteries)
give an example of carcinomas that can spread to the liver
colon, stomach, pancreatic via the portal vein
what causes the pain associated with acute inflammation?
1. stretching and distortion of tissues due to oedema and pus under high pressure in an abscess cavity
2. chemical mediators (eg bradykinin and prostaglandins) are also known to induce pain
what is the main source of histamine?
mast cells - histamine is stored in granules in their cytoplasm
what is the role of tissue macrophages in acute inflammation?
they secrete chemical mediators that attract neutrophil polymorphs
what is the role of the lymphatic system in acute inflammation?
lymphatic channels dilute and drain away oedematous fluid, therefore reducing swelling. antigens are also carried to lymph nodes for recognition by lymphocytes
what is the major role of neutrophil polymorphs in acute inflammation?
phagocytosis
define carcinogenesis
a multistep process in which normal cells become neoplastic cells due to mutations
what percentage of cancer risk is environmental/genetic?
85% environmental
15% genetic
5 host factors that can affect cancer risk
1. race
2. diet
3. constitutional factors (gender/age)
4. premalignant conditions
5. transplacental exposure
what causes skin cancer?
Exposure to UV light
what occupation makes people more susceptible to bladder cancer caused by aromatic amine exposure?
people who work in the rubber/dye industry
define neoplasm
an autonomous, abnormal, persistent new growth
what is a neoplasm composed of?
neoplastic cells and stroma
what is essential for neoplasm growth?
angiogenesis
what does a neoplasm release in order to initiate angiogenesis?
vascular endothelial growth factors
why does necrosis often occur in the centre of a neoplasm?
the neoplasm grows quickly and outgrows its vascular supply
what is the behavioural classifications of neoplasms?
benign, malignant or borderline
what are complications of benign neoplasms?
1. pressure on adjacent structures
2. obstruction to flow
3. transformation into malignant neoplasms
4. anxiety
what are complications of malignant neoplasms?
destroy surrounding tissue, blood loss due to ulceration, pain, anxiety
define carcinoma
malignant epithelial neoplasm - malignant tumour of epithelial tissue
define sarcoma
malignant connective tissue neoplasm
what is an adenoma?
benign tumour of glandular epithelium
what is a papilloma?
a non-glandular benign tumour
what is a leiomyoma?
benign smooth muscle neoplasm
what is a neuroma?
a benign neoplasm of nerves
what is a chondrosarcoma?
malignant neoplasm of cartilage
what is a lipsarcoma?
malignant neoplasm of adipose tissue
what is a melanoma?
malignant neoplasm of melanocytes
what is a lymphoma?
malignant neoplasm of lymphoid cells (immune cells)
what is a mesothelioma?
malignant neoplasm of mesothelial cells
is a carcinoma/sarcoma with a close resemblance to normal tissue classified as well differentiated or poorly differentiated?
well differentiated - these types of neoplasms are low grade and have a better prognosis
describe innate immunity
non-specific, instinctive, present from birth, first line of defence (eg cough reflex, mucus, enzymes and oils in tears)