5- Diffusion and Equilibrium Potentials
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
Ion Channel
integral membrane-spanning protein that selectively permits its passage of certain ions when open
selectivity filter
Determines whether the channel allows cations or anions to pass
-Which specific ions (e.g., Na+, K+, Ca2+. Cl-, etc.) can pass
What are ion channels controlled by?
gates
-Depending on position, they may be open or closed
Open gates
selected ion flows through the channel by passive diffusion down their concentration gradient; Continuous path between ECF and ICF
Closed gates
no movement of selected ion regardless of concentration gradient
Conductance
Probability that a channel is open
How many types of sensors control ion channel gates?
3: Voltage-gated, Second Messenger-gated, and Ligand-gated channels
Voltage-gated channels
-Gates are controlled by changes in membrane potential
-Activation gate opened by depolarization
-Inactivation gate closed by depolarization
Second Messenger-gated channels
-Gates controlled by changes in intracellular signaling molecule levels (cAMP, IP3)
-Sensors located on the intracellular side of the ion channel
Ligand-gated channels
-Gates controlled by hormones and neurotransmitters
-Sensors located on the extracellular side of the ion channel
Diffusion potential
The electrochemical potential difference generated across a membrane by a charged solute (cation/anion) diffusing down its concentration gradient
-ONLY generated if the membrane is permeable to a specific ion – regardless of how large the ion’s concentration gradient may be
What is the magnitude of the diffusion potential measured in?
Millivolts (mV)
-Depends on the size of the concentration gradient (driving force)
-The sign of the diffusion potential depends on the charge of the diffusing ion
How are diffusion potentials created?
by the movement of only a few ions
-They do not cause ion concentration changes in the bulk solution
What is the definition of equilibrium potential?
The diffusion potential that exactly balances (or opposes) the affinity for diffusion down a concentration gradient.
What happens when a cation diffuses down its concentration gradient?
It carries a positive (+) charge across the membrane that will slow and stop further ion diffusion.
What happens when an anion diffuses down its concentration gradient?
It carries a negative (-) charge across the membrane that will slow and stop further ion diffusion.
Electrochemical equilibrium
Chemical and electrical driving forces acting on the ion are equal and opposite; no further net diffusion occurs
Na+ Equilibrium Potential
-Na+ diffuses down its concentration gradient
-Net movement of positive charge to solution 2 causes a Na+ diffusion potential
--(Solution 2 becomes positive compared to solution 1)
--Solution 2 positivity prevents further Na+ diffusion
-Na+ equilibrium potential = Positivity balances further Na+ diffusion
-Electrochemical equilibrium = Electrical driving forces on Na+ are equal and opposite
Cl- Equilibrium Potential
-Cl- diffuses down its concentration gradient
-Net movement of negative charge to solution 2 causes a Cl- diffusion potential
--(Solution 2 becomes negative compared to solution 1)
--Solution 2 negatively prevents further Cl- diffusion
-Cl- equilibrium potential = Negativity balances further Cl- diffusion
-Electrochemical equilibrium = Electrical driving forces on Cl- are equal and opposite
Nernst Equation
Used to calculate the equilibrium potential for an ion at a given concentration difference across a membrane
-Calculated for one ion at a time
-Converts an ion concentration difference into a voltage
-R = Gas constant; T = Absolute temperature; F = Faraday constant
-Multiply by 2.3 converts natural logarithm to log10

How is membrane potential expressed?
as intracellular potential relative to extracellular potential
-(e.g., a transmembrane potential difference of -70 mV means “cell interior negative 70 mV”
Uncharged solutes
Net diffusion driving force is the concentration difference of the solute across the cell membrane
Charged solutes
Net diffusion driving force is the difference between the actual, measured membrane potential (Em) and the ion’s calculated equilibrium potential (Ex)
Negative driving force
(Em is more negative than ion equilibrium potential)
-The ion will enter the cell if it is a cation and leave if it is an anion
Positive driving force
(Em is more positive than ion equilibrium potential)
-The ion will leave the cell if it is a cation and enter the cell if it is an anion
Equal driving force
(Em is equal to the ion equilibrium potential)
-There is NO driving force and NO net movement of the ion in either direction
Ionic current (current flow)
Movement of an ion across a cell membrane
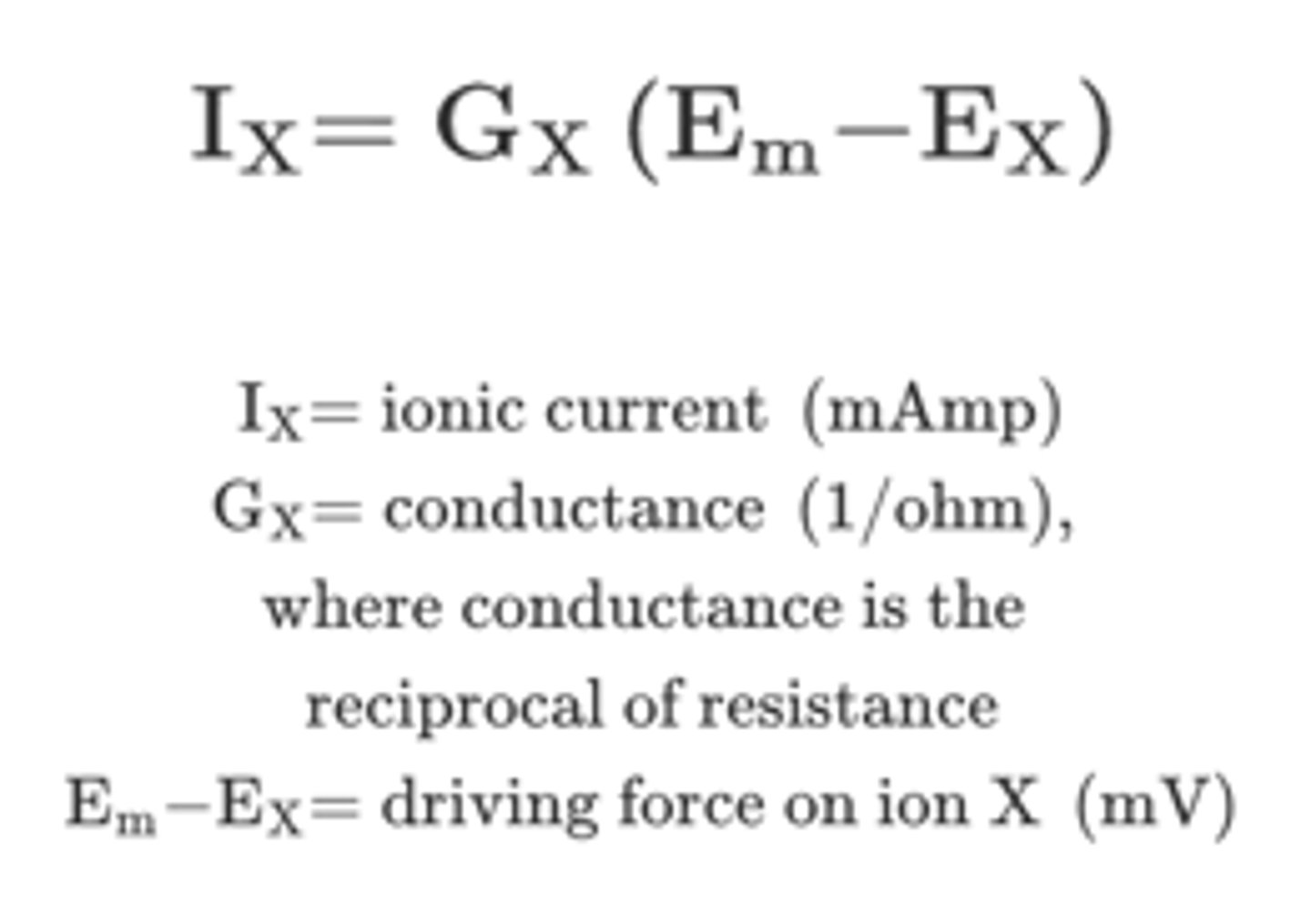
What two conditions assist ions in moving through ion channels?
-There is an ionic driving force
-The membrane has a conductance (open channel) for the ion
Ionic current direction
determined by the direction of the driving force
Ionic current magnitude
determined by the size of the driving force and the conductance of the ion
if the driving force is high
the greater the current flow
When positive charges move out of a cell, it is called
outward current
What is resting membrane potential?
The potential difference existing across the membrane of excitable cells in the period between action potentials.
What establishes the resting membrane potential?
Diffusion potentials.
How do ions affect the resting membrane potential?
Each ion attempts to drive the membrane potential toward its own equilibrium potential.
Which ions contribute most to the resting membrane potential?
Ions with the highest permeability (conductances) at rest.
Which ions contribute little to the resting membrane potential?
Ions with the lowest permeability.
What is the resting potential for most excitable cells?
between -70 to -80 mV
What is the resting membrane potential close to?
the equilibrium potentials for K+ and Cl-
What is the permeability of K+ and Cl- ions at rest?
high
What is the resting membrane potential far from?
the equilibrium potentials for Na+ and Ca2+
What is the permeability of Na+ and Ca2+ ions at rest?
low
Why is it necessary to maintain the K+ concentration gradient?
To establish the resting membrane potential (e.g., Na+/K+ ATPase pump)