2.2 - Chemical Reactions and Enzymes
Chemical reaction: a process that changes one set of chemicals to another.
Changes chemical bonds between atoms in elements or compounds.
Can occur slowly (over years) or quickly (milliseconds).
Matter and energy are conserved in all chemical reactions
A + B → AB
A + B are the reactants (elements or compounds that enter into chemical reactions)
AB is the product (elements or compounds produced by chemical reactions
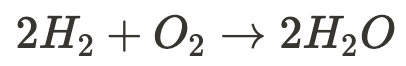
All chemical reactions involve energy (absorbed or released as light, heat, or sound)
- endothermic reactions absorb energy from the environment and require an energy source
- exothermic reactions release energy to the environment and can occur spontaneously
All reactions, whether they absorb or release energy overall, need energy to begin
- Reactants must collide with enough energy for bonds to break and form
- The energy of the reactants alone is not enough to cause a reaction
Activation energy: The difference in energy between that of the reactants and the amount needed for the reaction to occur.
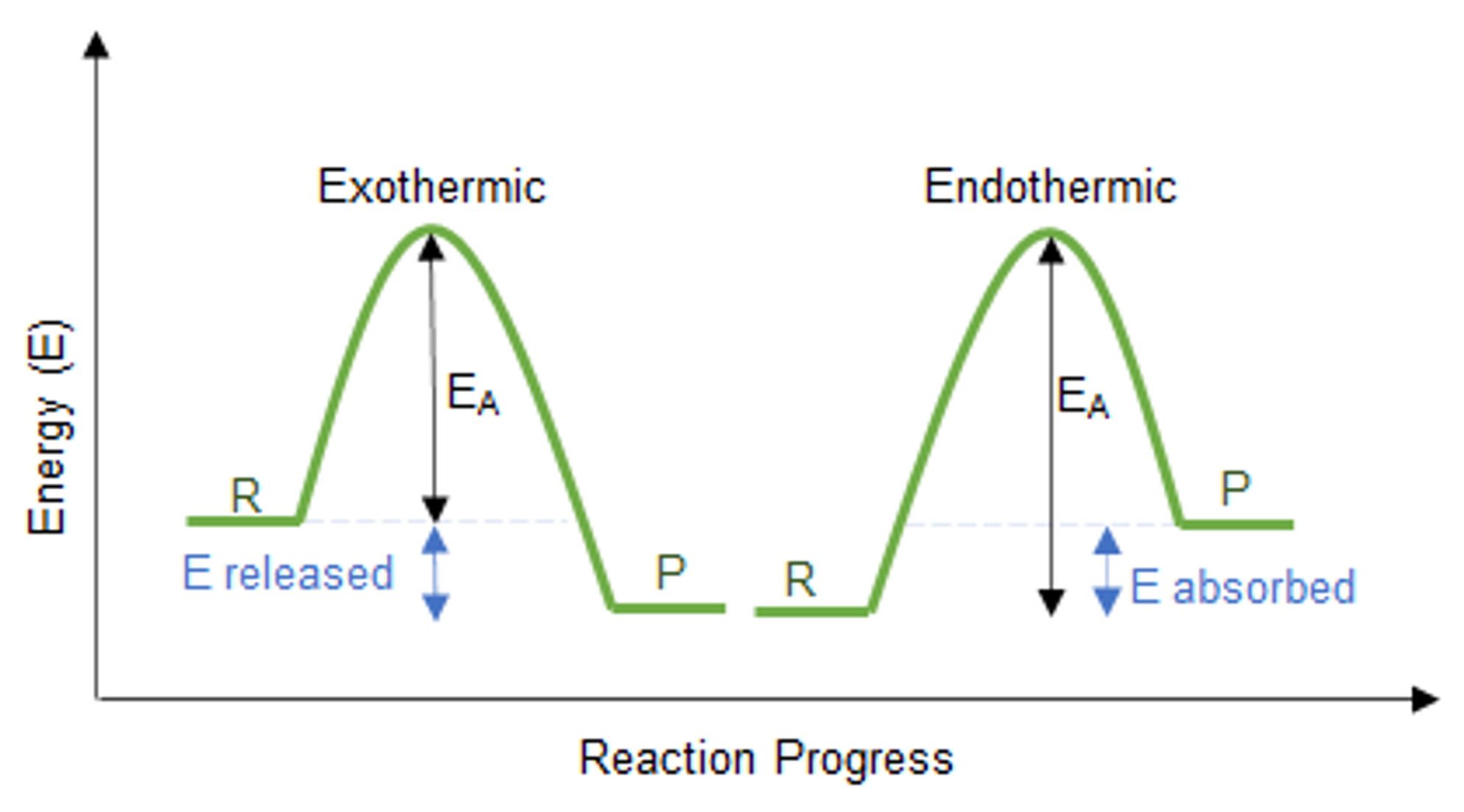
Catalysts lower the activation energy needed for a chemical reaction to occur, increasing the rate of a reaction.
Catalysts are unchanged by reactions they catalyze
Catalysts are not used as reactants or created as products of reactions
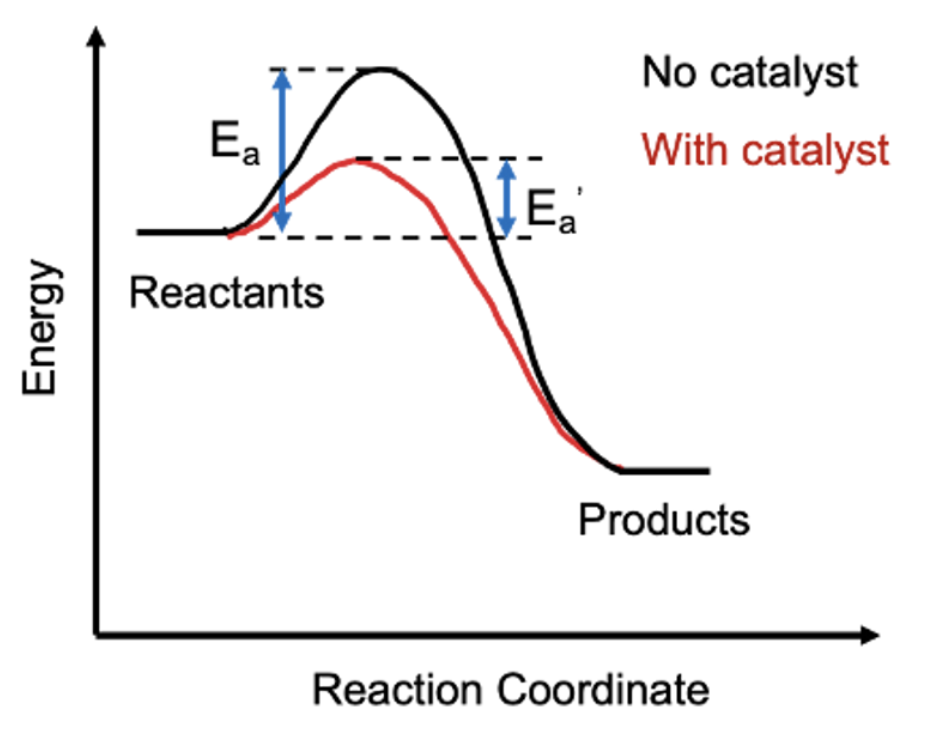
Enzymes: proteins that act as catalysts for chemical reactions within cells
- Catalyze only one type of chemical reaction
- Named after the reaction they catalyze
- Usually ending with the suffix “-ase”
Examples: Lactase (cleaves lactose) and DNA Polymerase (makes DNA polymers)
Reactants in enzyme-catalyzed reactions are called substrates
Enzymes have an active site where substrates are brought together to react
Enzymes only need to bind to a few types of substrates so bonding between them is highly specific (like a lock and key)
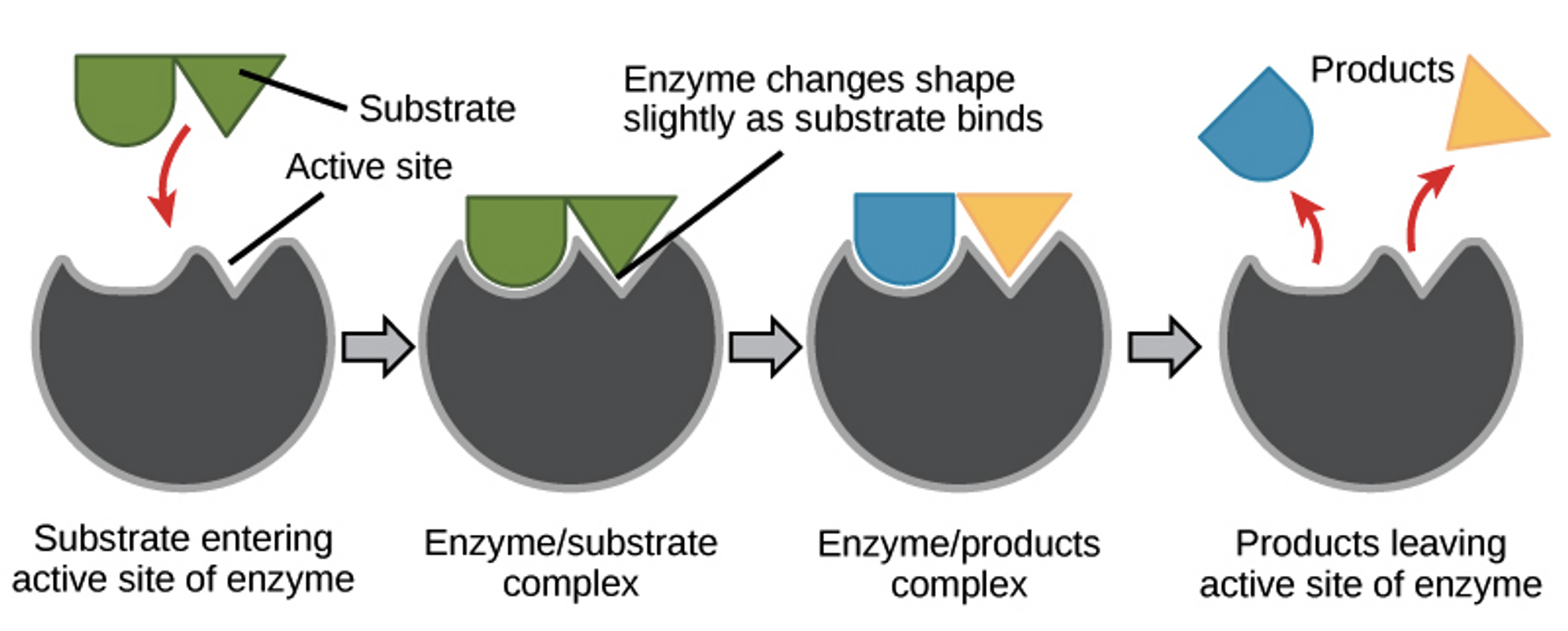
Enzymes work best under specific conditions:
- pH, temperature, concentration of substrate
- Presence of regulatory molecules:
- Cofactors: needed to activate or enhance enzyme function
- Inhibitors: can decrease or stop an enzyme’s activity
- Competitive inhibitors: binds to an active site and physically blocks substrate
- Non-competitive inhibitors: binds elsewhere and changes the shape of the active site so substrate cannot bind
Enzymes in human cells work best at body temperature (37C)
- Unfold at high temperatures (denature)
- Unfolding changes the shape of active sites, preventing enzymes from catalyzing reactions
pH can be used to regulate enzymes
enzyme inhibitors can be used to treat diseases
enzymes can be given as supplements (e.g. lactase)