bonds
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
8 Terms
Metallic bond properties
do not dissolve in water
conduct electricity
bendable and hard solids
made entirely of metal atoms
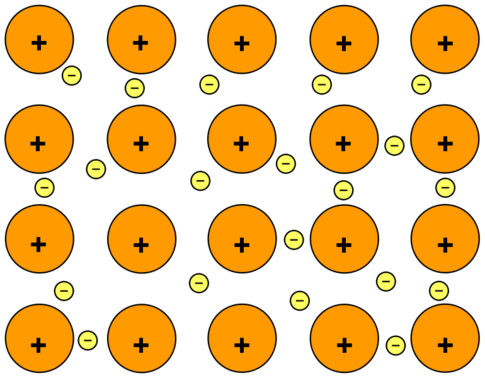
Metallic bond valence electrons movement
Free to move about the substance
Network Covalent Properties
do not dissolve in water water
do not conduct electricity
extremely hard solids
made entirely of nonmetal and metalloid atoms
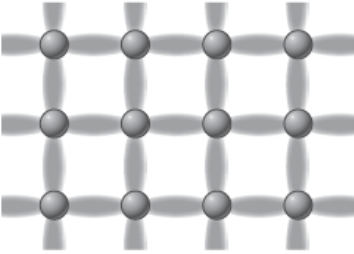
Network covalent valence electrons movement
Valence electrons connect atoms with each other in all directions, like a grid or network.
Ionic bond properties
dissolve in water
conduct electricity with dissolved
brittle solids
made of metal and nonmetal atoms combined
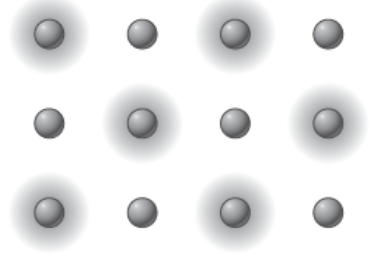
Ionic bond valence electrons movement
Metal atoms “give up” their valence electrons to nonmetal atoms.
Molecular covalent bond properties
some dissolve in water, some do not
do not conduct electricity
some are liquids or gases
made entirely of nonmetal atoms
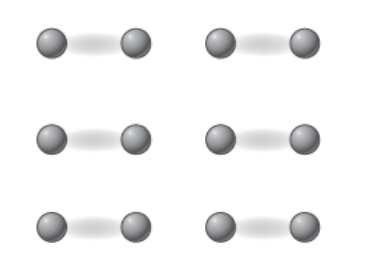
Molecular covalent bond valence electrons movement
Valence electrons are shared between some atoms. This creates small stable units within the substance.