Chapter 13 & 15
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
Because of its relevance in Distillation
Why is VLE the most studied type of phase equilibrium?
activity coefficient
the _______________ is a correction factor that accounts for deviations from ideal solution behavior by relating a component's actual chemical potential to what it would be in an ideal solution at the same composition.
liquid-phase property
The activity coefficient is understood to be a ______________________

Formula in Gamma/Phi Formulations
Ideal gas model for gas state
Ideal solution model for liquid state
What are the two simplest models in VLE?
Raoult’s law
Ideal gas and ideal solution models are combined in the simplest treatment of vapor/liquid equilibrium in what is known as ___________________
1
This then produces the equation for Raoult’s Law
If the vapor phase is assumed to be in its ideal-gas state and the liquid phase is assumed to be an ideal solution, both Φi and γi approach what value?
Francois Marie Raoult
Who proposed Raoult’s Law?
Only for Ideal mixtures
The ideal-gas-state assumption means that Raoult’s law is limited in application to low to moderate pressures.
system are chemically similar.
it can be applied only to species of known vapor pressure
this requires each species to be “subcritical,” i.e., at a temperature below its critical temperature.
What are the limitations of Raoult’s Law?
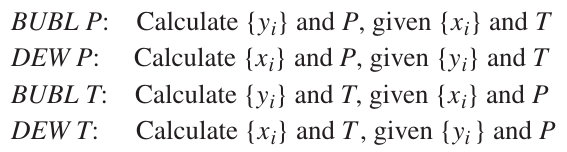
4 types of Bubble and Dew Point Calculations
In modified Raoult's law, what is "modified" is the addition of activity coefficients (γᵢ) to correct for non-ideal solution behavior
What is modified in Modified Raoult’s Law?
Chemical potential must be equal across all phases
Partial molar Gibbs energy should be minimized
Constant T and P
Fugacity of species i should be equal across all phases
Criterion for VL Equilibrium
Henry's law states that the partial pressure of a component in the vapor phase is directly proportional to its mole fraction in the liquid phase. Henry's constant - typically used for dilute solutions where one component (usually the solvent) follows Raoult's law while the solute follows Henry's law.
What is Henry’s Law?
Gibbs/Duhem
Henry’s law is related to the Lewis/Randall rule through the ______________ equation
Henry’s law applies to a species as it approaches infinite dilution in a binary solution, and the Gibbs/Duhem equation insures validity of the Lewis/Randall rule for the other species as it approaches purity.
Henry’s law applies to a species as it approaches infinite dilution in a binary solution, and the Gibbs/Duhem equation insures validity of the Lewis/Randall rule for the other species as it approaches purity.
Henry’s law applies to a species as it approaches infinite dilution
Lewis/Randall rule applies for the other species as it approaches purity
Gibbs/Duhem equation insures validity
In Henry’s Law/Lewis-Randall Rule binary calculations,
___________ applies to a species as it approaches infinite dilution
_____________ applies for the other species as it approaches purity
__________________ insures validity
Redlich-Kister Expansion
Margules Model
Van Laar Models
Different GE models
Margules Equation
Simplified version of Redlich-Kister
Redlich Kister
Pure empirical approach with temperature-dependent parameters
5
Usually, how many parameters are used in Redlich Kister equation?
2, A12 and A21
How many adjustable parameters in Margules?
Van laar equation
they do not incorporate an explicit temperature dependence of their parameters, though this can be supplied on an ad hoc basis.
2
How many parameters in Van laar?
local composition
Theoretical developments in the molecular thermodynamics of liquid-solution behavior are often based on the concept of ________________________
G. M. Wilson in 1964
Who introduced the concept of Local Compositions?
2
How many parameters in Wilson equation?
Non-Random Two-Liquid
What is the meaning of NRTL?
3
How many parameters are used in NRTL?
They are specific to a particular pair of species
Independent of composition and temperature
Describe the parameters used in NRTL
UNIQUAC
UNIFAC
These are the local composition models of greater complexities
Universal Quasichemical
Meaning of UNIQUAC
UNIQUAC Functional-group Activity Coefficients
Meaning of UNIFAC
ΔG and its first and second derivatives are continuous functions of x1, and the second derivative is positive.
At fixed temperature and pressure, a single-phase binary mixture is stable if and only if
Constant T and P
Fugacity of species i is equal across all phases
Equilibrium criteria for LLE
Crystallization
Phase behavior involving the solid and liquid states is the basis of important separation processes like
Assume ideal-solution behavior for both phases
Assume ideal-solution behavior for the liquid phase and complete immiscibility for all species in the solid state
Two cases in Solid-Liquid Equilibrium
sublimation curve
Solid/vapor equilibrium for a pure species is represented on a PT diagram by the _________________