CH 5 gen chem
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
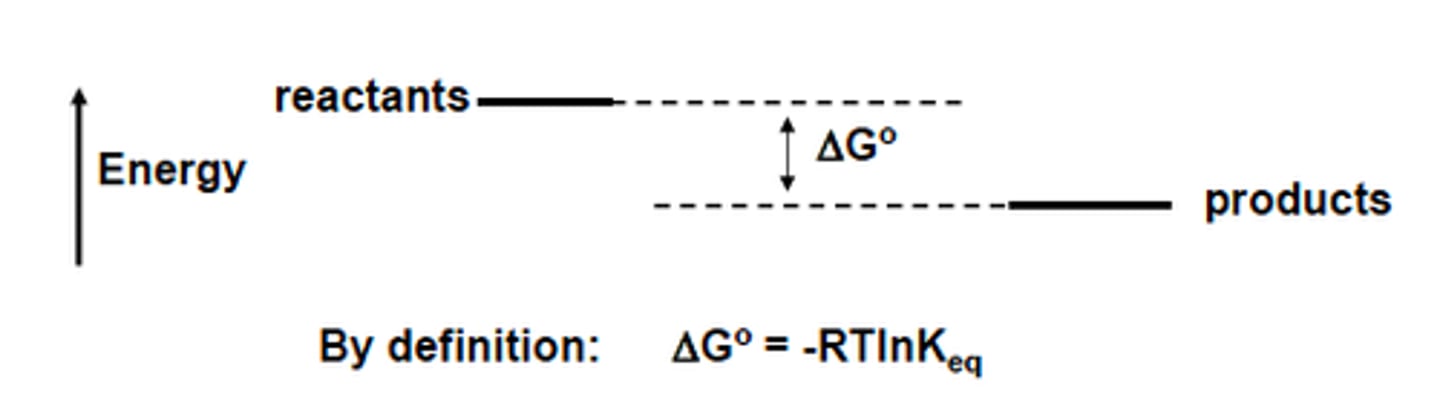
Gibbs free energy (Delta G) determines what? Give the related reaction types
Determines whether or not a reaction is spontaneous.
G > 1 = endergonic (nonspontaneous)
G=0, equilibrium
G < 1 = exergonic (spontaneous)
intermediate
-molecule in the mechanism that doesn't appear in the overall reaction
rate-determining step
the slowest step in a reaction mechanism, prevents the overall reaction from proceeding any faster than that slowest step
Chemical Kinetics
the area of chemistry that is concerned with reaction rates and reaction mechanisms
Collision theory
States that a reaction rate is proportional to the number of effective collisions between the reacting molecules.
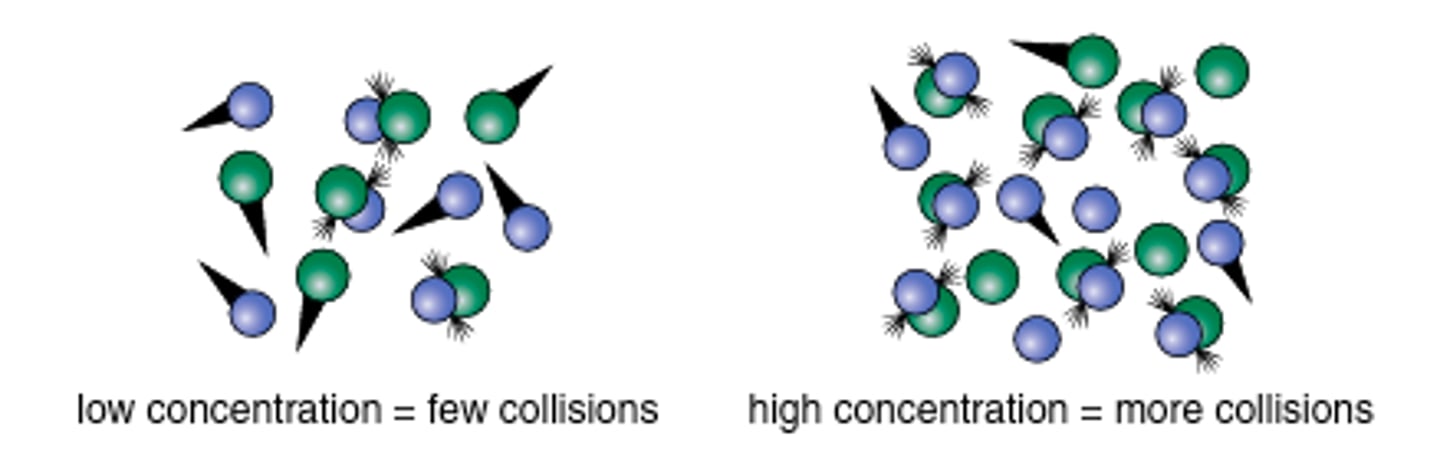
How do molecules react? ie what makes a collision effective
1) Must be in proper orientation
2) Must have enough KE to overcome activation energy
Activation energy
The minimum energy colliding particles must overcome in order to react.
Arrhenius equation
write the equation
define the variables
describe how the change in variables affects the rate constant
Mathematical way of representing collision theory.
low activation energy and high temp make the negative exponent smaller in magnitude, which increases the rate constant k
*A is the frequency or pre-exponential factor and e^(-Ea/RT) is the fraction of collisions that have enough energy to react (i.e., have energy greater than or equal to the activation energy Ea) at temperature T.
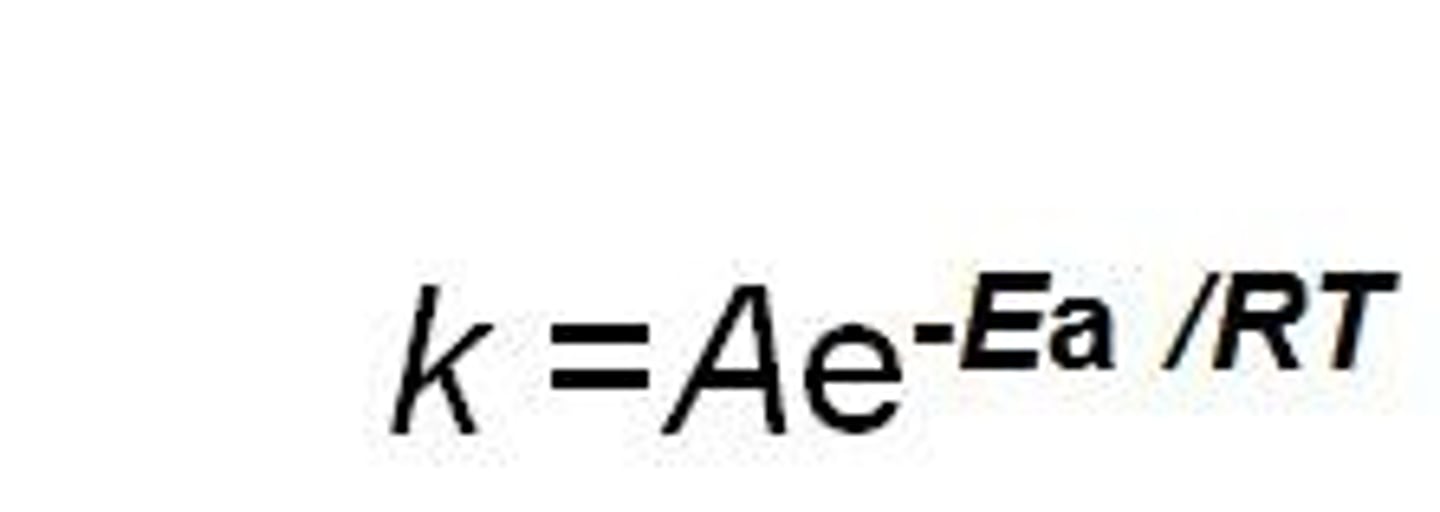
frequency factor(attempt frequency) and how can it be increased
a term in the Arrhenius equation that is related to the frequency of collision and the probability that the collisions are favorably oriented for reaction
- can be increased by increasing the number of molecules in a vessel
Transition state theory
States that molecules form a transition state or activated complex during a reaction in which the old bonds are partially broken and new bonds partially formed.
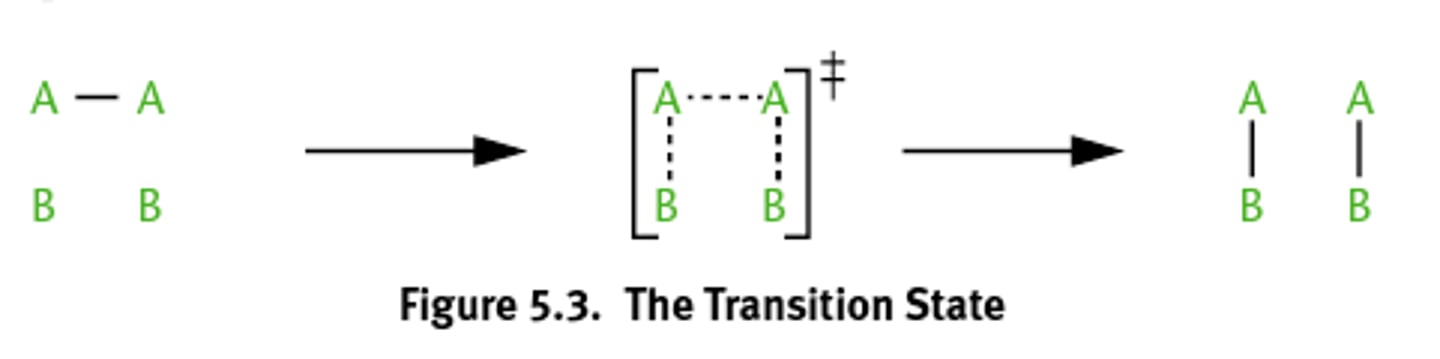
Transition state(activated complex)
1) Highest point on a free energy reaction diagram.
2) Reaction can proceed toward products or revert to reactants.
3) old bonds broken and new bonds formed here.
4) the energy required to reach this state is the activation energy
highest energy relative to reactants and products
positive delta G
endergonic
negative delta g
exergonic
Factors affecting reaction rates:
1) Increasing [reactant] (except 0 order) because more effective collision per time.
2) Increasing temperature will increase reaction rate because particles KE will increase.
3) Changing medium will increase or decrease reaction rate, depending on how reactants interact with the medium (soluble or not?), generally POLAR SOLVENTS are preferred because molecular dipole tends to polarize the bonds of the reactants lengthening and weakening them permitting the reaction to occur faster
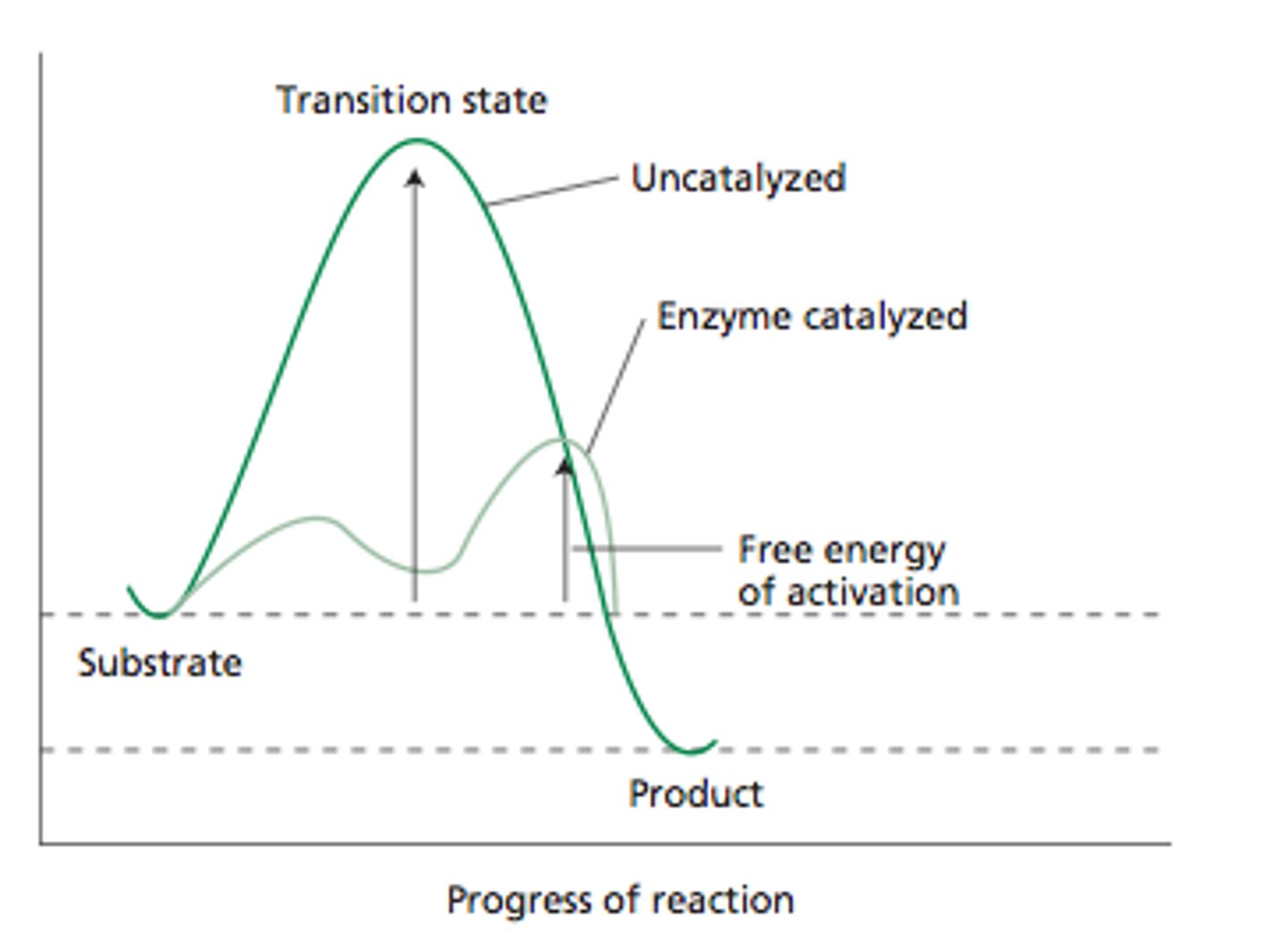
4) Adding a catalyst (lowers activation energy)
![<p>1) Increasing [reactant] (except 0 order) because more effective collision per time.</p><p>2) Increasing temperature will increase reaction rate because particles KE will increase.</p><p>3) Changing medium will increase or decrease reaction rate, depending on how reactants interact with the medium (soluble or not?), generally POLAR SOLVENTS are preferred because molecular dipole tends to polarize the bonds of the reactants lengthening and weakening them permitting the reaction to occur faster</p><p>4) Adding a catalyst (lowers activation energy)</p>](https://knowt-user-attachments.s3.amazonaws.com/7a6f557f-8543-4498-8fe7-4beb8f196e43.jpg)
catalyst
substance that speeds up the rate of a chemical reaction
- interacts with reactants
- decreases the activation energy
- no impact on free energy
only make spontaneous reactions move more quickly toward equilibrium
as useful as catalysts are in biological and nonbiological
systems, catalysts are not miracle workers: they will not transform a
nonspontaneous reaction into a spontaneous one; they only make
spontaneous reactions move more quickly toward equilibrium.
basically the transition state energy is reduced but the free energy of the reactants and products do not chnage
Homogeneous catalyst
Same phase as reactants
Heterogeneous catalyst
different phase than reactants
Reaction rates are meas in terms of
Measured in terms of rate of disappearance of a reactant or appearance of a product.
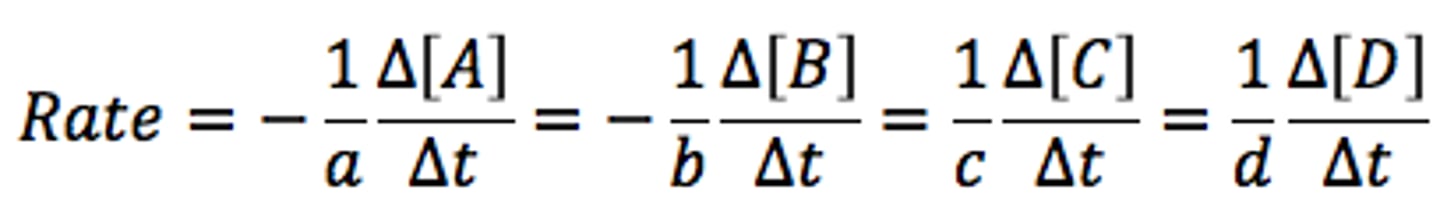
Rate laws
Equations that indicate how the rate of the reaction, R, depends on the concentration (M) of the reactants
values of m and n are almost never equal to the stoichiometric coefficients
rate law is different than equilibrium expression
rate law expression only includes the reactants
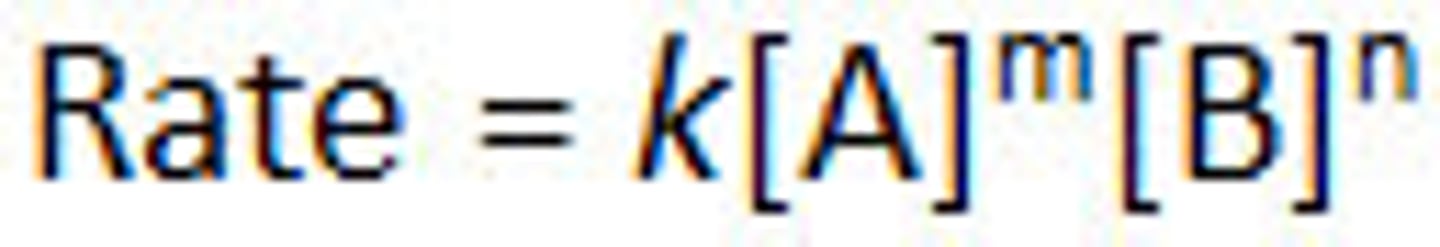
law of mass action
the equilibrium constant expression
How is the rate order of a reaction determined
1) Does not match with stoichiometric coefficients
2) Determined from experimental data
3) Sum of all individual rate orders in the rate law
Zero-order reaction
Have a constant rate that does not depend on the concentration of the reactant(s): rate=k[A]^0[B]^0 --> rate=k
1) Only affected by changing temp. or adding catalyst
2) Concentration vs Time curve = straight line; slope of line = -k
units M/s
![<p>Have a constant rate that does not depend on the concentration of the reactant(s): rate=k[A]^0[B]^0 --> rate=k</p><p>1) Only affected by changing temp. or adding catalyst</p><p>2) Concentration vs Time curve = straight line; slope of line = -k</p><p>units M/s</p>](https://knowt-user-attachments.s3.amazonaws.com/ae59c03a-f9da-42c6-89a1-a04a8186eec3.jpg)
First-order reaction
Have a non constant rate that depends on the concentration of one reactant, such that doubling the concentration of that reactant results in the doubling of the rate of formation of the product: rate=k[A]^1 or rate=k[B]^1
molecule undergoes change without interaction with another molecule
1) Concentration vs Time curve = nonlinear
2) Slope of ln [A] vs time plot is -k for first order
unit s^-1
![<p>Have a non constant rate that depends on the concentration of one reactant, such that doubling the concentration of that reactant results in the doubling of the rate of formation of the product: rate=k[A]^1 or rate=k[B]^1</p><p>molecule undergoes change without interaction with another molecule</p><p>1) Concentration vs Time curve = nonlinear</p><p>2) Slope of ln [A] vs time plot is -k for first order</p><p>unit s^-1</p>](https://knowt-user-attachments.s3.amazonaws.com/18e42dd8-fdc0-464f-98e9-dba84066dec7.jpg)
Second-order reaction
Have a non-constant rate that depends on the concentration of a reactant(s): rate=k[A]^1[B]^1 OR rate=k[A]^2 OR rate=k[B]^2
collision between 2 molecules
1) Concentration vs Time curve of second-order reaction is nonlinear.
2) The slope 1/[A] vs time plot = k for second-order
units M^-1s^-1
![<p>Have a non-constant rate that depends on the concentration of a reactant(s): rate=k[A]^1[B]^1 OR rate=k[A]^2 OR rate=k[B]^2</p><p>collision between 2 molecules</p><p>1) Concentration vs Time curve of second-order reaction is nonlinear.</p><p>2) The slope 1/[A] vs time plot = k for second-order</p><p>units M^-1s^-1</p>](https://knowt-user-attachments.s3.amazonaws.com/d4f20c0d-0e56-42c0-952b-2d40ce41d281.jpg)
Broken-order reactions
Those with non-integer orders
Mixed-order reactions
Those that have a rate order that changes over time.
how to interpret mixed-order rate law:
rate= k1 [C][A]^2/k2 + k3[A]
larger value for [a] at the beginning of the reaction is that k3[A] >>K2 and the rxn will appear to be first order with respect to A.
at the end of the reaction when [A] is low, k2>>k3[a] making the reaction appear second order with respect to a
Rate order problem + explanation
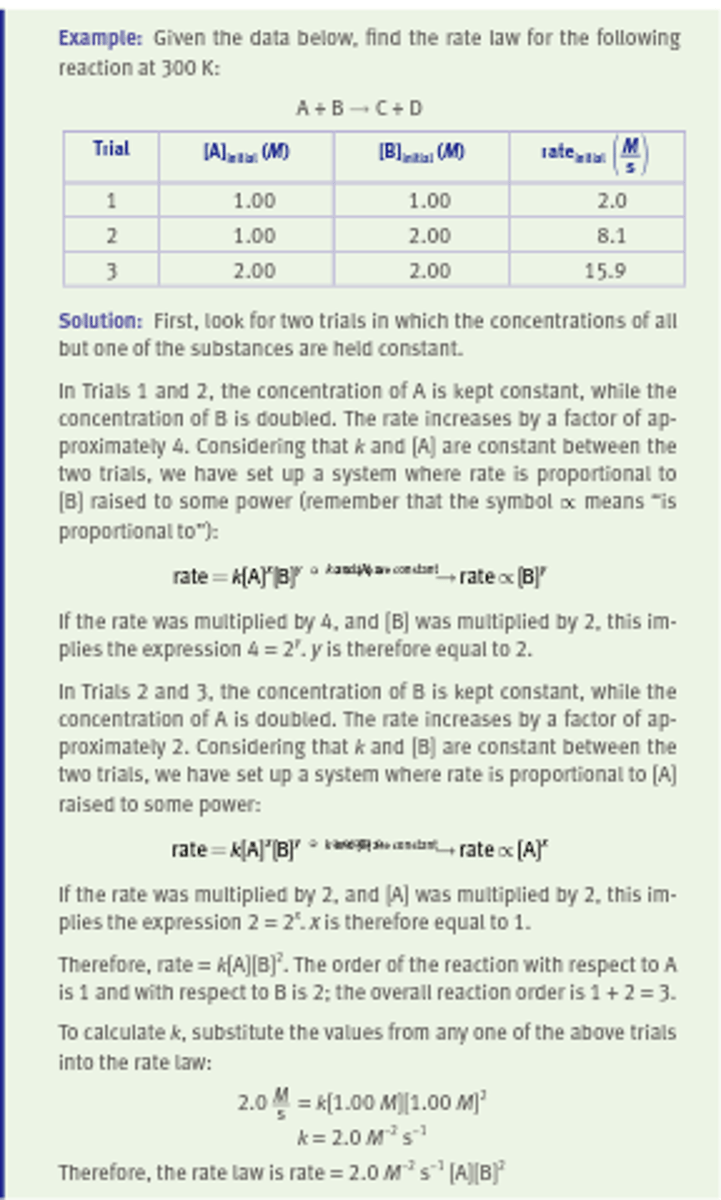
Rate Questions
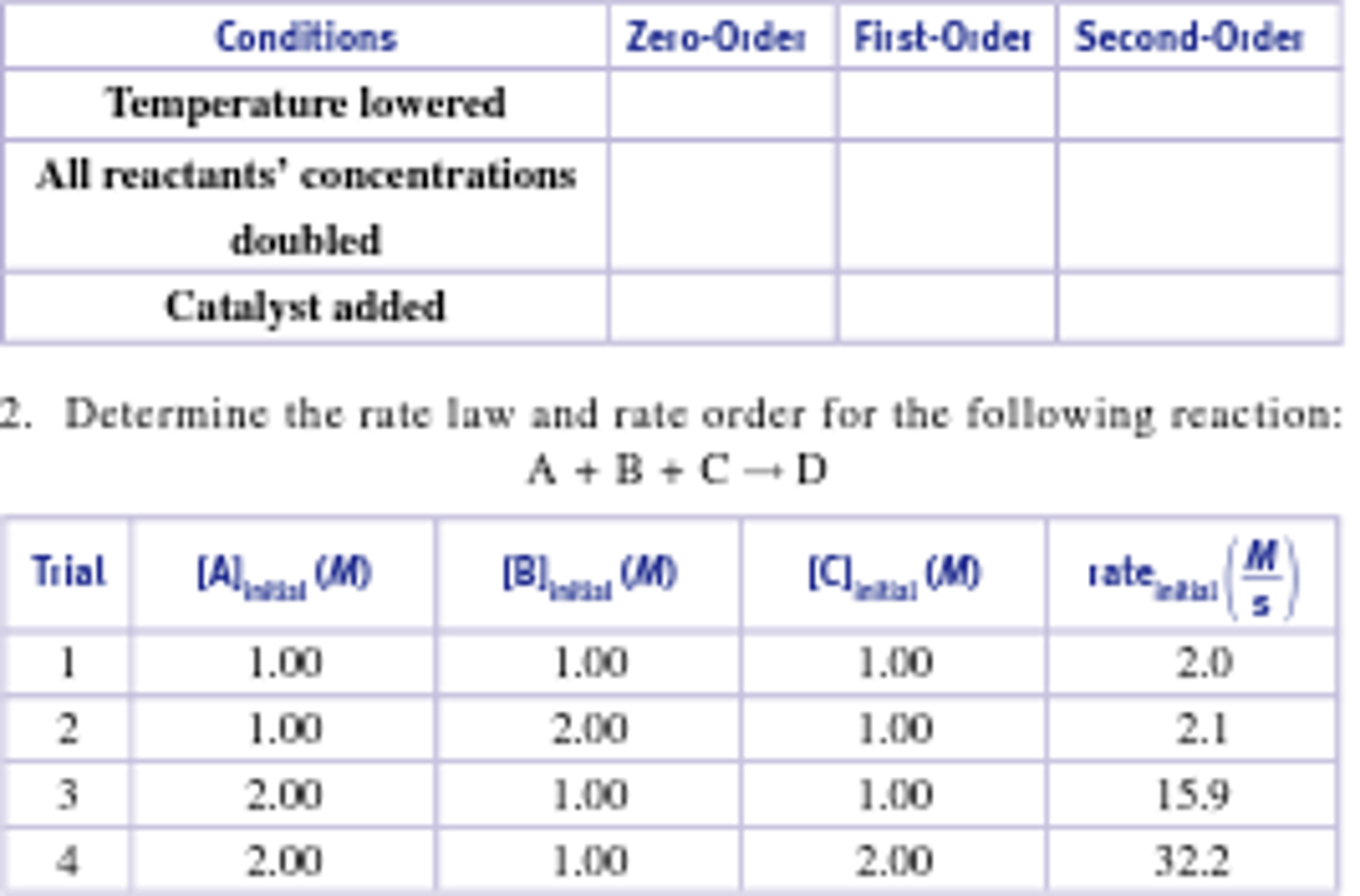
Rate Answers
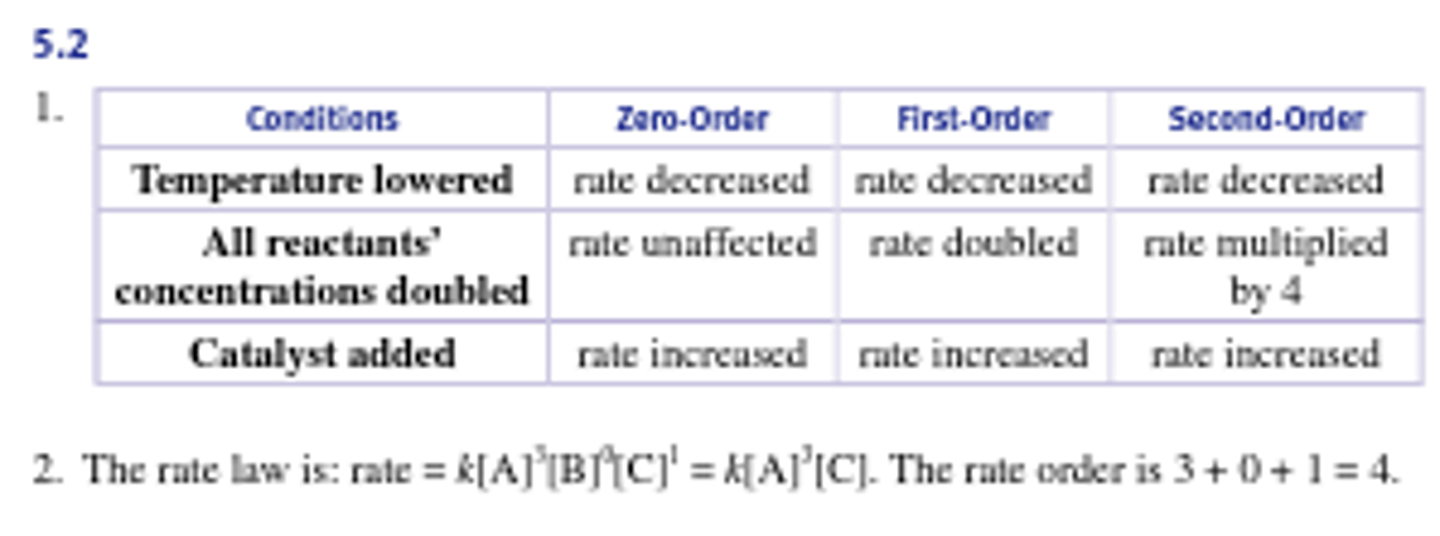
What would increasing the concentration of reactants accomplish in a solution containing a saturated catalyst?
The reaction rate would be unaffected because saturated solutions with a catalyst have a maximum turnover rate
True or False
In a reaction where the rate = k [NO2][Br2]
the amount of NO2 consumed is equal to the amount of Br2 consumed
False because the exponents in the rate law are unrelated to the stoichiometric coefficients, so NO2 and Br2 could have any stoichiometric coefficients in the original reaction and still be a second-order reaction
What is the overall order of the reaction if the rate law is:
rate = k [A]^0 [B]^2 [C]^1
3 because the overall order of the reaction is the sum of the individual orders in the reaction
a reactant in a second-order reaction at a certain temperature is increased by a factor of 4. By how much is the rate of the reaction altered?
A. unchanged
b. increased by factor of 4
c. increased by factor of 16
d. it cannot be determined from the information given
D. we don't know if its [A]2[B]0 or [A]1[B]1 or others so we cannot determine
The following system obeys second-order kinetics
2NO2 -> NO3 + NO (slow)
NO3 + CO -> NO2 + CO2 (fast)
what is the rate law?
rate=k[NO2]2
slow step is rate-determining step and related to concentrations of reactants in rate-determining step not overall reaction so NO2 only compound that should be included, stem tells us its second-order so squared