MCAT Physics Review - Ch. 3+4: Thermodynamics; Fluids
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Zeroth Law of Thermodynamics
Temperature
Heat
Thermal equilibrium
Fahrenheit/Celsius/Kelvin
Absolute zero
Third law of thermodynamics
Material Thermal Expansion
Coefficient of linear expansion
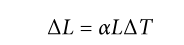
Liquid Thermal Expansion
Coefficient of Volumetric Expansion
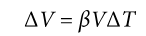
Systems
System
Surroundings
Isolated vs. Closed vs. Open Systems
Bomb calorimeter
State functions (memorize)
First Law of Thermodynamics
Heat
Work
Second law of thermodynamics
Heat transfer
Conduction
Convection
Radiation
Specific heat
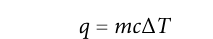
Heat of Transformation
Microstates
Heat of transformation (i.e., latent heat)
Melting/fusion
Freezing/solidification
Boiling/evaporation/vaporization
Condensation
Sublimation
Deposition
Melting point
Heat of fusion + Heat of vaporization
Boiling point
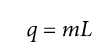
Thermodynamic processes
Isothermal
Adiabatic
Isovolumetric/isochoric
Isobaric
Entropy
Second law of thermodynamics
Natural process
Irreversible vs. reversible reactions
Unnatural process
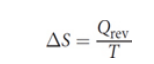
Characteristics of Fluids and Solids
Fluids
Solids
Shear (tangential) forces
Density
Density
Specific gravity

Pressure
Pascal (Pa)
mmHg = torr
Absolute pressure
Absolute (hydrostatic) pressure
Incident/ambient pressure
Gauge pressure
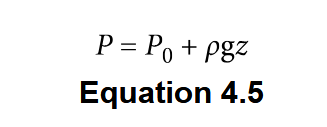
Hydrostatics
Pasca’s principle
Hydraulic systems
Isobaric process
Archimedes’ principle
Buoyancy

Molecular Forces in Liquids
Surface tension
Cohesion
Adhesion
Meniscus
Backward meniscus
Fluid Dynamics
Viscosity (eta)
Viscous drag
Inviscid
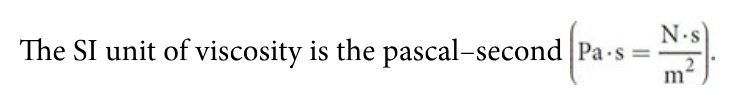
Laminar and Turbulent Flow
Laminar flow
Poiseuille’s law
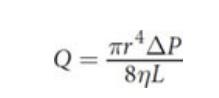
Turbulence and Speed
Turbulent flow
Eddies
Critical speed
Boundary layer
Reynolds number
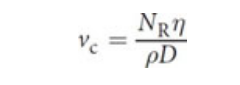
Streamlines
Flow rate
Linear speed
Continuity equation
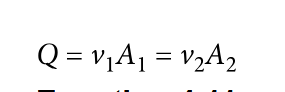
Bernoulli’s Equation
Dynamic pressure
Energy density
Static pressure
Pitot tubes
Venturi flow meter
Venturi effect
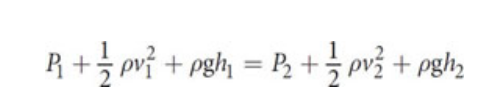
Fluids in Physiology
Circulatory system
Closed loop
Pulse
Respiratory