Solutions Part 2
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
what is a colligative property
when 4 physical properties are affected in the same way as solutes
what are the 4 main colligative properties
vapour pressure lowering
freezing point depression
boiling point elevation
osmotic pressure
why is osmotic pressure really important
as it contributes to the flow of nutrients through biological cell walls
what is the osmotic pressure
when a solution is seperated from its solvent by a membrane then the excess pressure which has to be applied to prevent the flow of solvent is known as
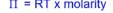
how do you calculate the osmotic pressure
USING THE VAN’T HOFF EQUATION
pi = RT x molarity
R = gas constant
T = temprature
what is the van’t hoff equation used for
to calculate osmotic pressure
what is hydrostatic pressure
pressure exerted by a column of aqueous solution or water
what is reverse osmosis
when a pressure (greater than osmotic pressure) forces the solvent through the membrane
what is dialysis
a seperation process based on equal rates of passage of solute and solvent through microporou membranes
what is an effect of osmosis
when a plant is dehydrated it will wilt
how can greater osmotic pressure affect red blood cells
water will pass out to reduce chemical potential across cell membrane ( cell shrinks)
how can lower osmotic pressure affect red blood cells
water will enter the cell ( cell swells) and eventually breakdown
what is an isotonic solution
when there is no net flow of solvent when the solutions are separated by a biological membrane
when the phase change is vapour to solid what is it called
deposition