Nuclear Energy
0.0(0)
0.0(0)
New
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
Alpha decay
The nucleus emits an alpha particle (2 protons and 2 neutrons, same as a helium nucleus).
Effect:
The atom’s mass number decreases by 4.
The atomic number decreases by 2.
Example:
Uranium-238 → Thorium-234 + alpha particle.
2
New cards
Beta decay
A neutron in the nucleus changes into a proton and releases a beta particle (an electron).
Effect:
The mass number stays the same.
The atomic number increases by 1 (because of the extra proton).
Example:
Carbon-14 → Nitrogen-14 + beta particle.
3
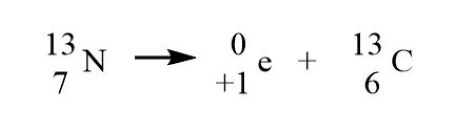
New cards
Positron emission
emits positron, charge goes down.
4
New cards
Electron Capture
takes in electron, charge goes down