Functional Groups and Carbonyl Derivatives - Vocabulary Flashcards
1/20
Earn XP
Description and Tags
Vocabulary flashcards covering functional groups and carbonyl/carboxyl derivatives as described in the notes.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Alkane
Lacks other common functional groups; contains only C—C single bonds.
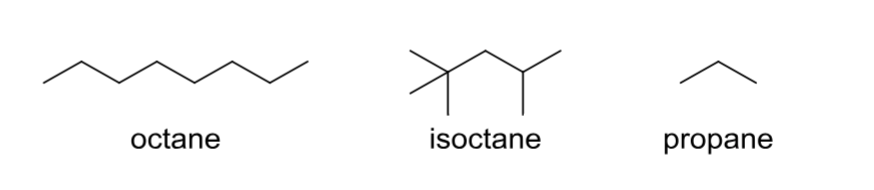
Alkene
Contains at least one C—C double bond.
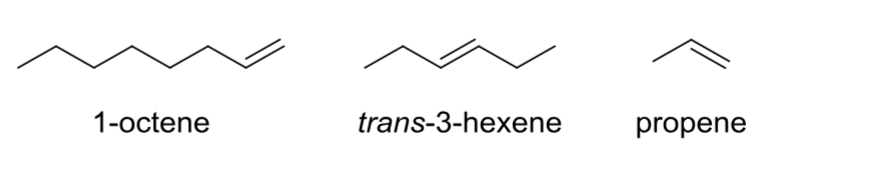
Alkyne (acetylene)
Contains at least one C—C triple bond.
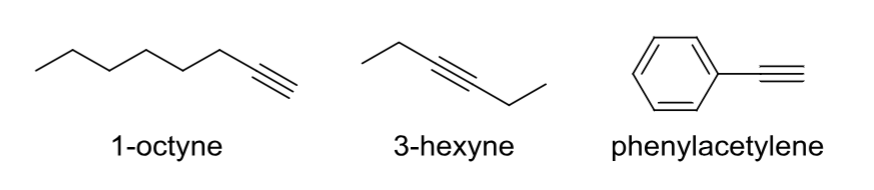
Aromatic
Cyclic, all atoms have a p orbital with pi electrons (2, 6, 10, …); not an alkene.
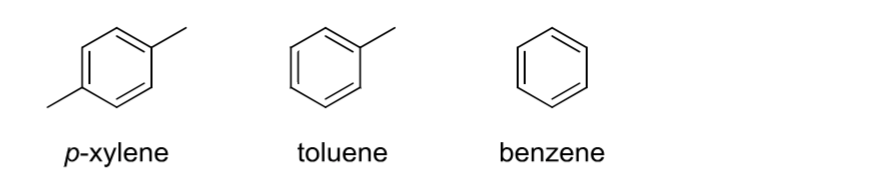
Alkyl Halide
A carbon group with a (Group 17)halogen attached.
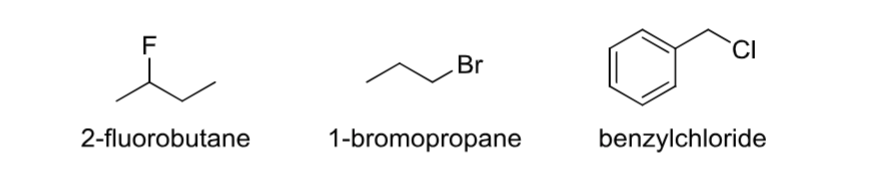
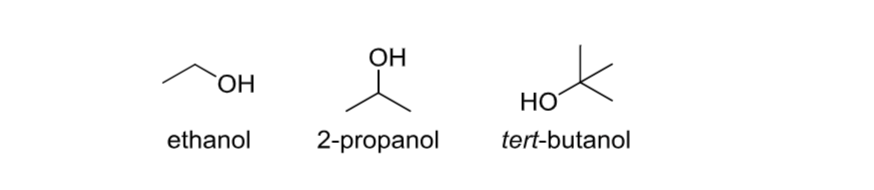
Alcohol
Contains an -OH group.
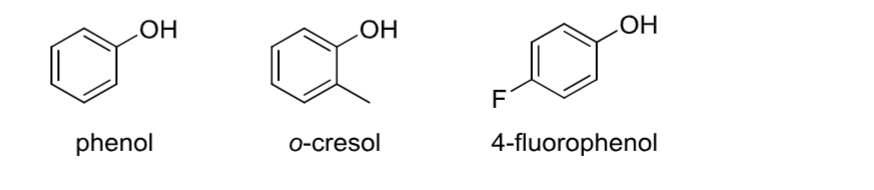
Phenol
Contains an -OH group attached to an aromatic ring.
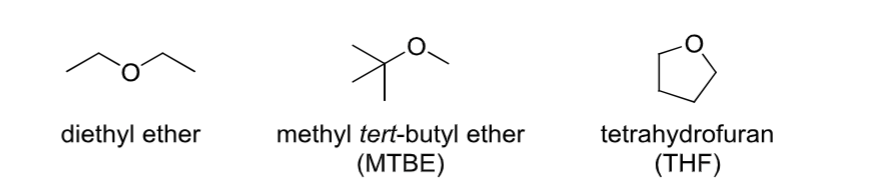
Ether
Contains an R-O-R group.
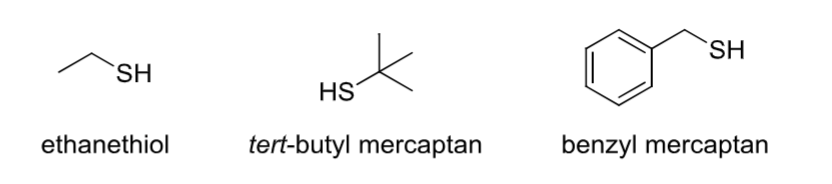
Thiol
Contains an -SH group.
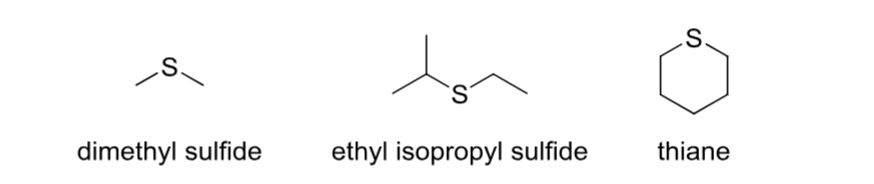
Sulfide (thioether)
Contains an R-S-R group.
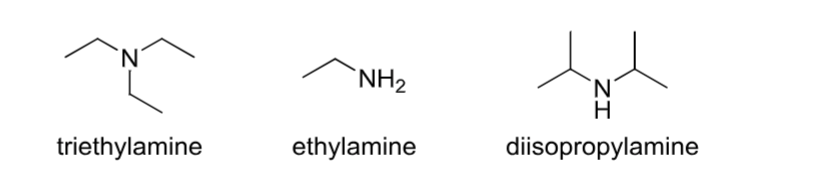
Amine
Contains an R3N group where R is hydrogen or a carbon group.
Ketone
A carbonyl bound to two carbon substituents.
Aldehyde
A carbonyl bound to a carbon and a hydrogen.
Imine
C=NR is like a ketone/aldehyde but O is replaced by NR.
Acetal
A carbon bound to two –OR groups.
Carboxylic Acid
A carbonyl bound to a carbon and an –OH group (–COOH).
Ester
A carbonyl bound to a carbon and an –OR group.
Amide
A carbonyl bound to a carbon and an –NR2 group.
Acid chloride
A carbonyl bound to a carbon and an –Cl group.
Anhydride
A carbonyl bound to a carbon and an –O–CO–R group (two acyl groups connected through oxygen).
Nitrile
A carbon triple bonded to nitrogen (–C≡N).