Chapter 9: VSEPR Structures WITH ANGLES
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
How are electron domains arranged?
Arranged to minimize repulsive energy
How many electron domains is a single bond? Double? Triple?
Single, Double, and Triple Bonds = ALL WORTH ONE ELECTRON DOMAIN
How many electron domains are two electrons?
One lone pair = one electron domain
Which has greater repulsion: lone pairs or bond pairs?
Lone pairs > bond pairs
When drawing 3D shapes, what do lines, wedges, and dashed lines mean?
Lines: Bonds are plane to the paper
Wedges: Bonds are coming out of the paper, towards you
Dashed Lines: Bond pointing away, towards the back
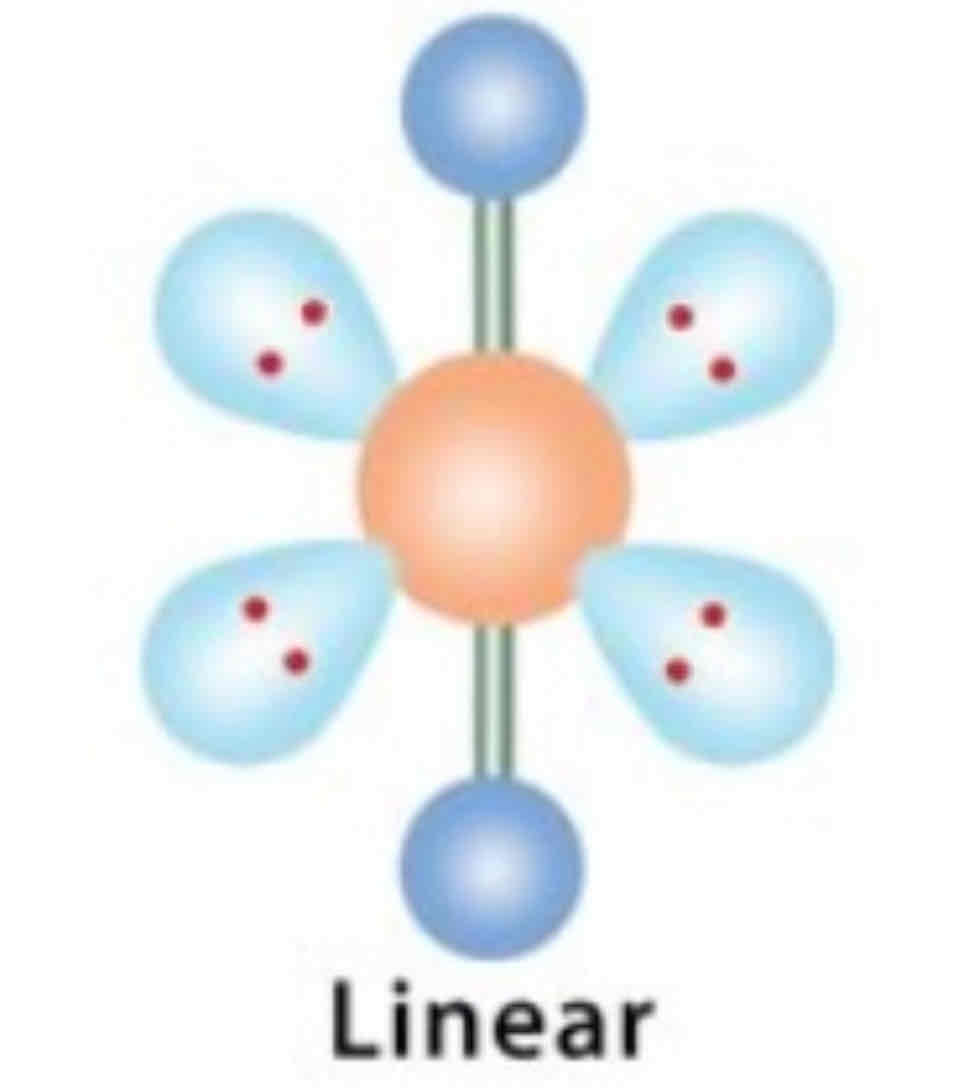
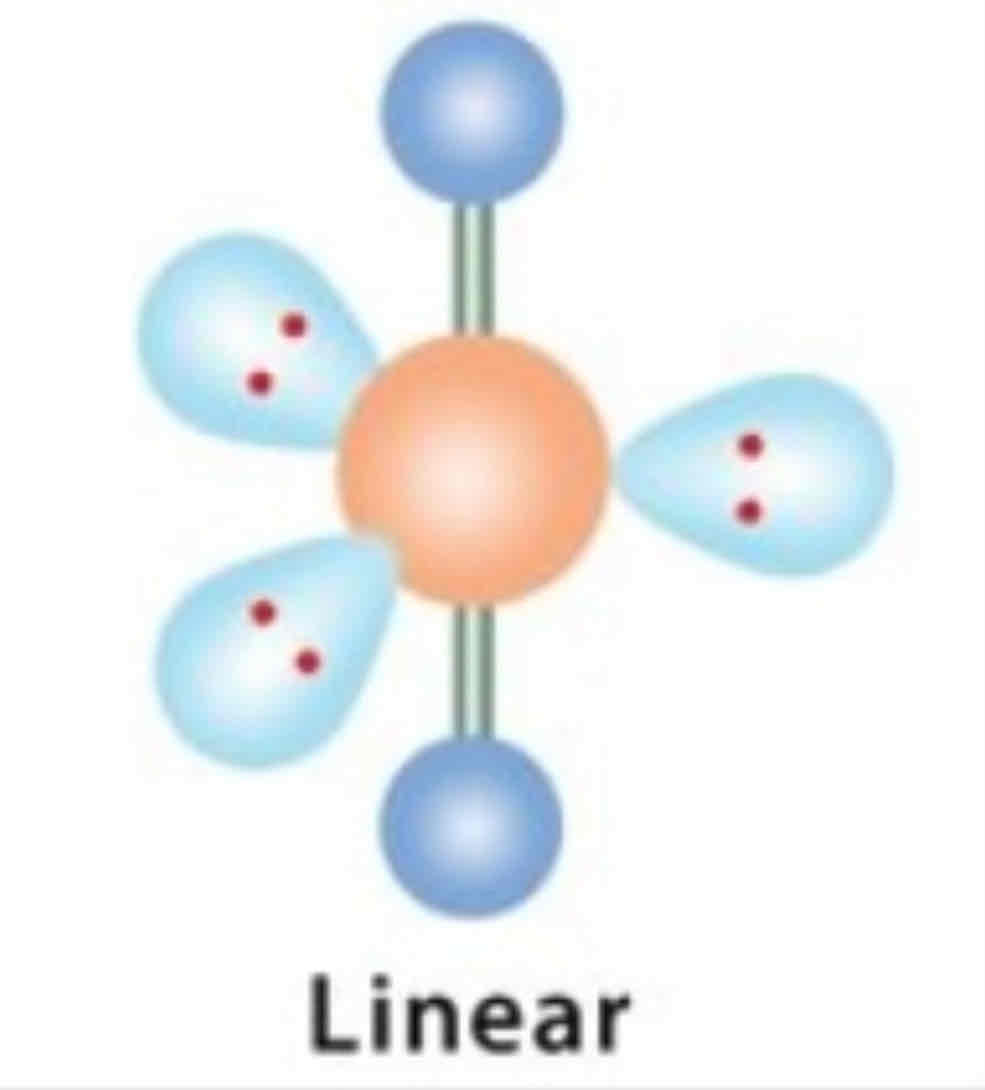
EDG and MG: 2 bonded pairs
EDG: Linear
MG: Linear
180 degrees
EDG and MG: 3 bonded pairs
EDG: Trigonal Planar
EDG: Trigonal Planar
120 degrees

EDG and MG: 2 bonded pairs, 1 lone pair
EDG: Trigonal Planar
MG: Bent
Two greater than 120, one less than 120
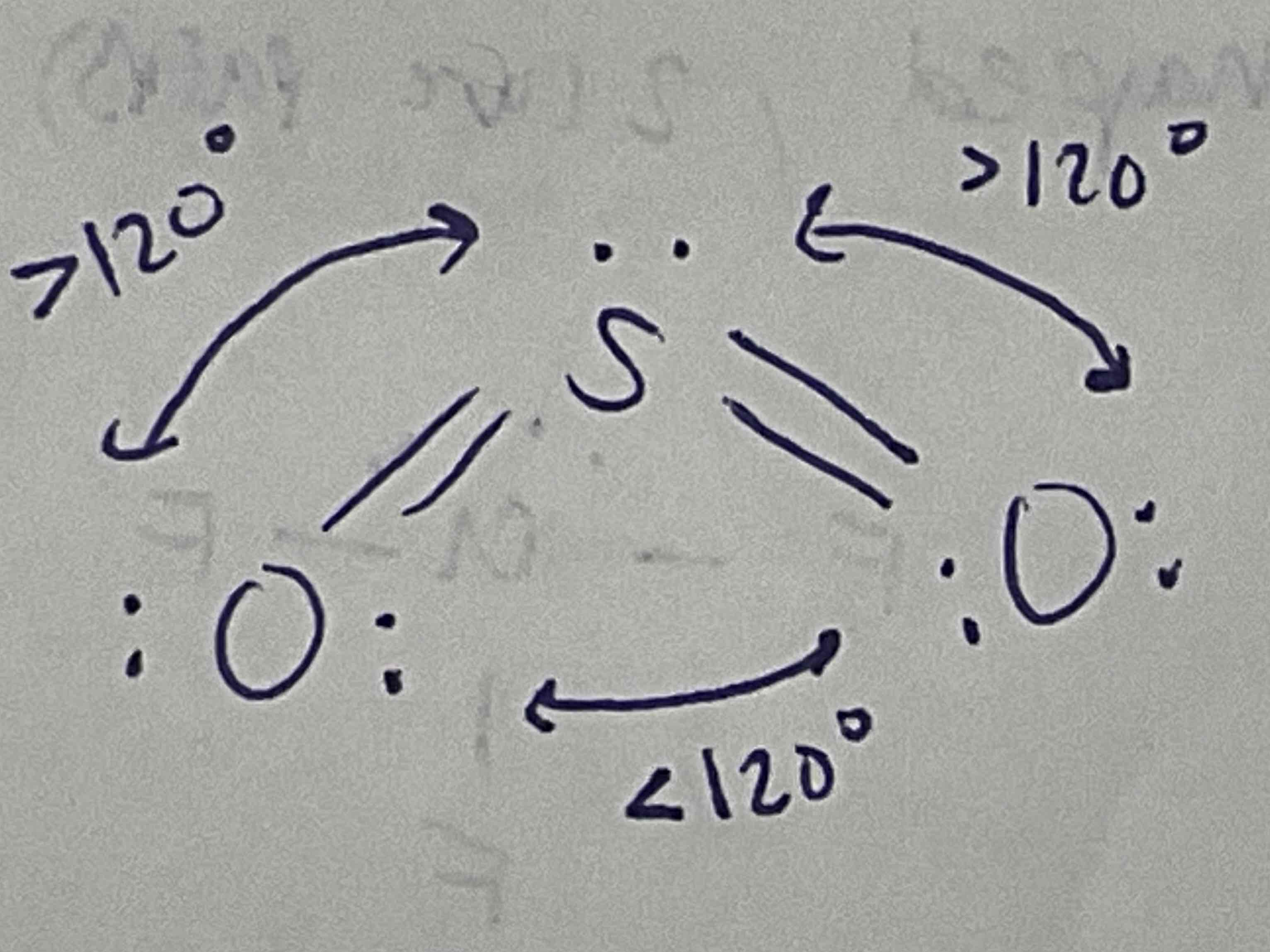
EDG and MG: 4 bonded pairs
EDG: Tetrahedral
MG: Tetrahedral
All 109.5 degrees
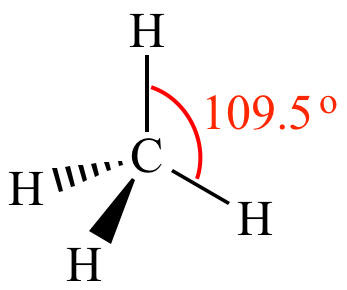
EDG and MG: 3 bonded pairs, 1 lone pair
EDG: Tetrahedral
MG: Trigonal Pyramidal (has a triangle base)
Less than 109.5, around 107 degrees

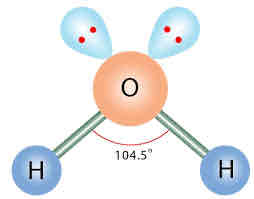
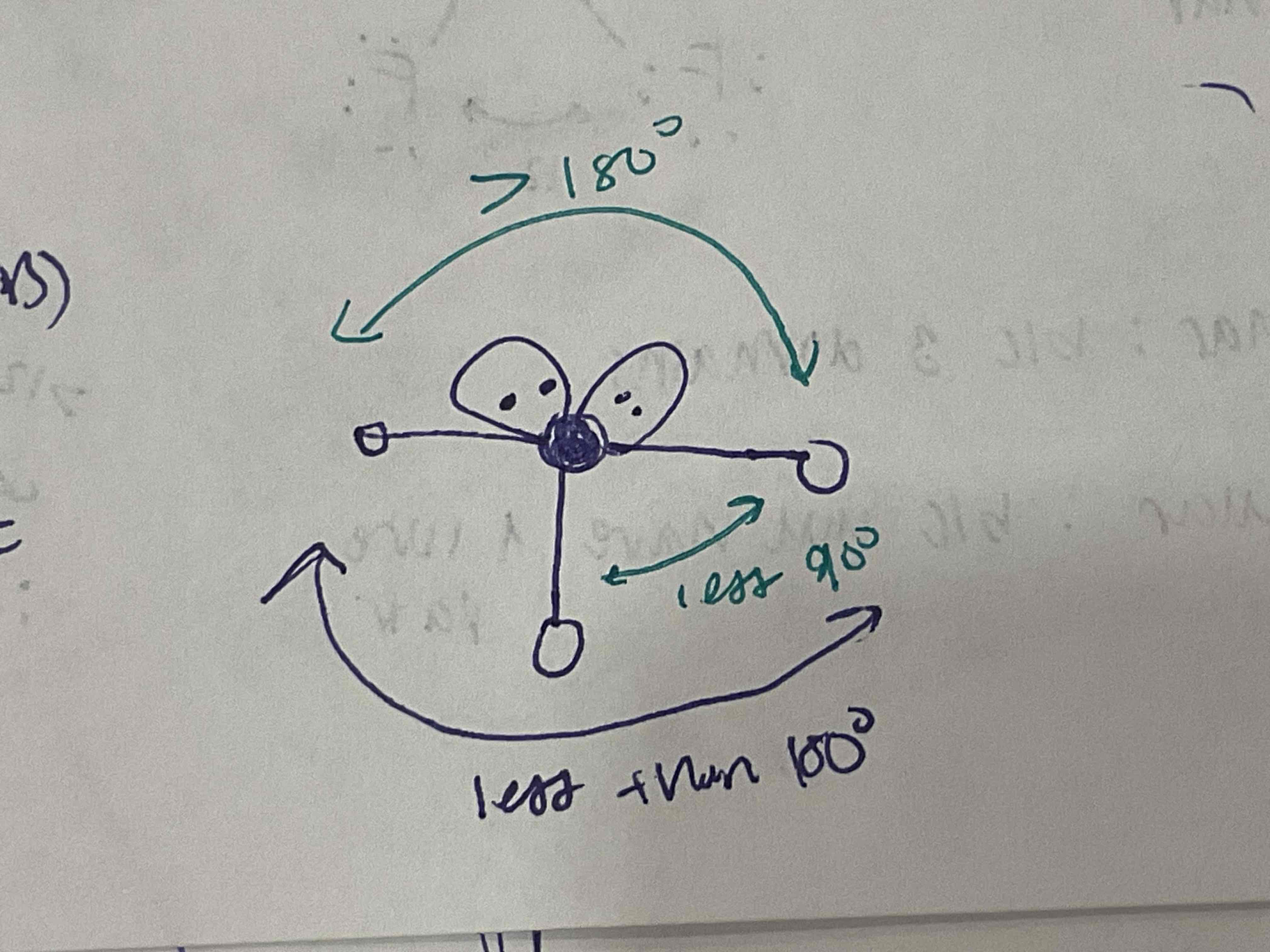
EDG and MG: 2 bonded pairs, 2 lone pairs
EDG: Tetrahedral
MG: Bent/Angular (ex. H2O)
Less than 109.5, approx 104.5. Other side is more than 180
EDG and MG: 5 bonded pairs
EDG: Trigonal Bipyramidal
MG: Trigonal Bypyramidal
Equatorial: 120 degrees. Equatorial to axis: 90 degrees

EDG and MG: 4 bonded pairs, 1 lone pairs
EDG: Trigonal Bipyramidal
MG: See saw
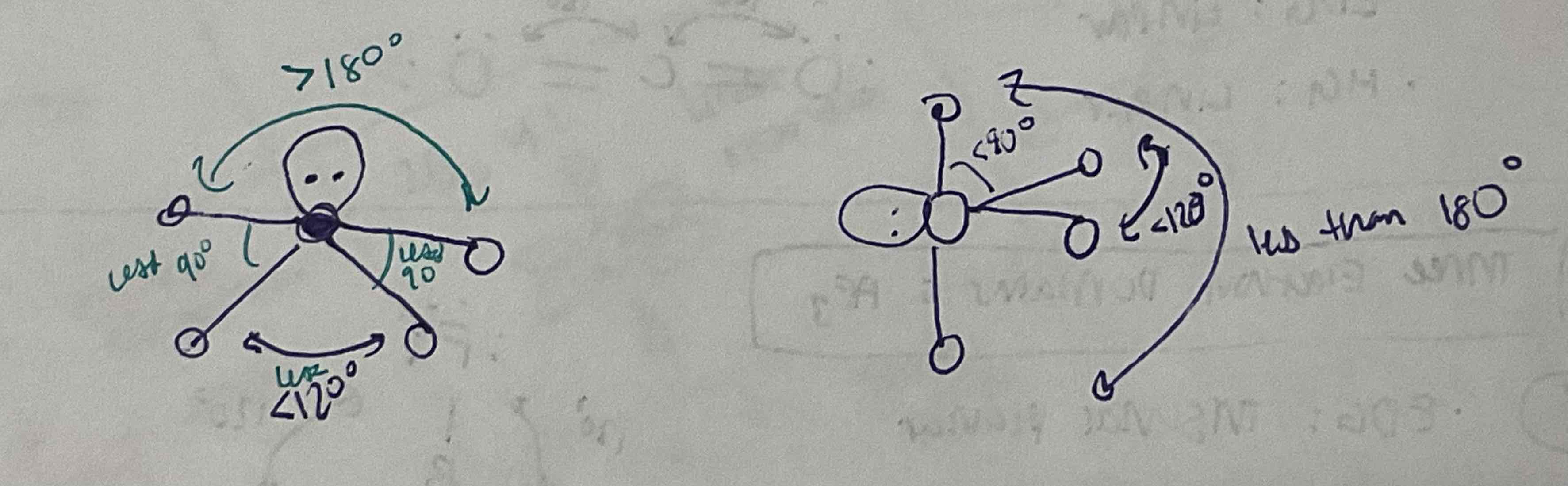
EDG and MG: 3 bonded pairs, 2 lone pairs
EDG: Trigonal Bipyramidal
MG: T-shaped
EDG and MG: 2 bonded pairs, 3 lone pairs
EDG: Trigonal Bypyramidal
MG: Linear
Axis to axis = 180
EDG and MG: 6 bonded pairs
EDG: Octahedral
MG: Octahedral
180 axis. 90 equatorial.
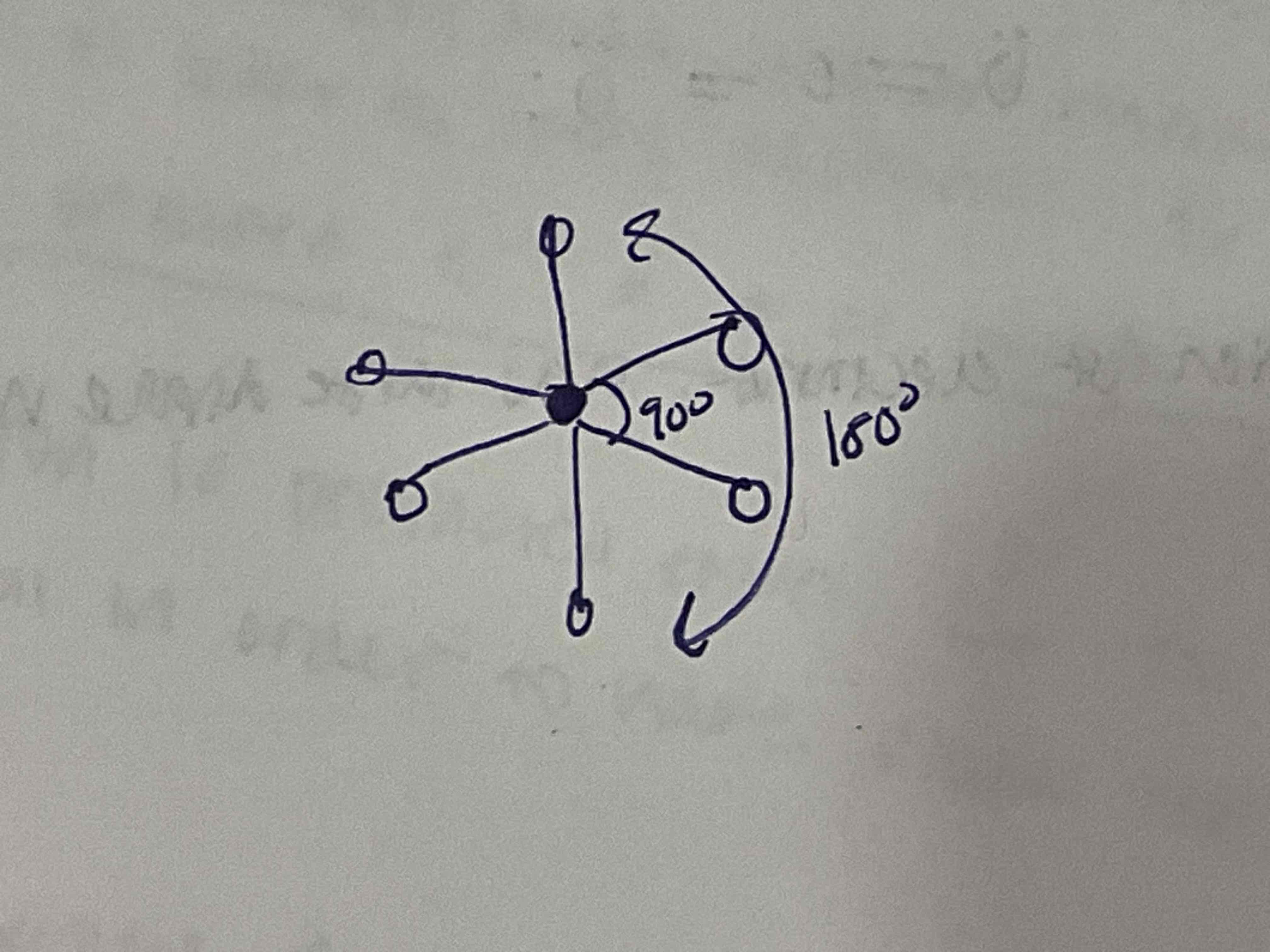
EDG and MG: 5 bonded pairs, 1 lone pairs
EDG: Octahedral
MG: Square Pyramidal
Equatorial: less than 90 degrees
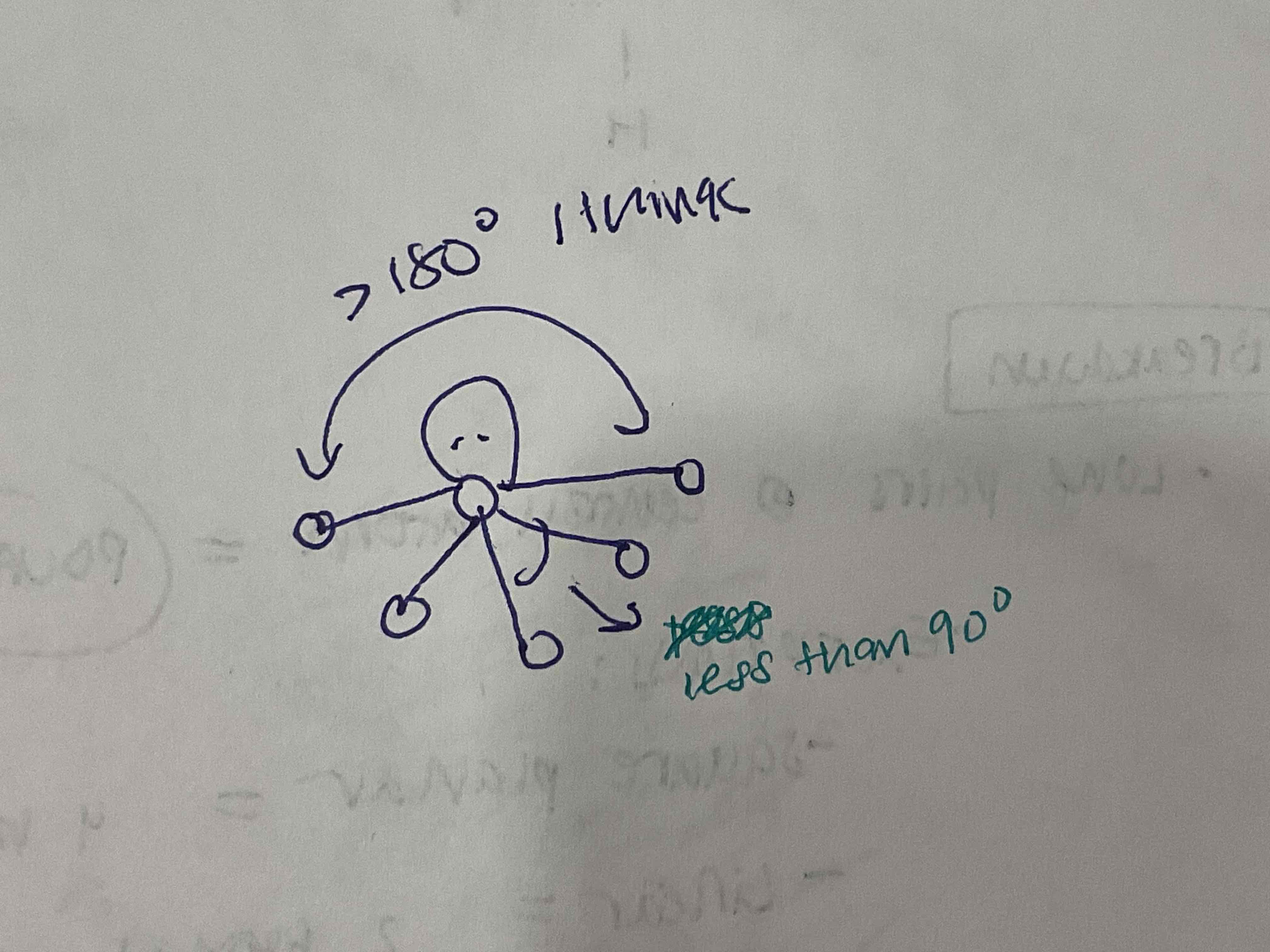
EDG and MG: 4 bonded pairs, 2 lone pairs
EDG: Octahedral
MG: Square Planar
All 90 degrees
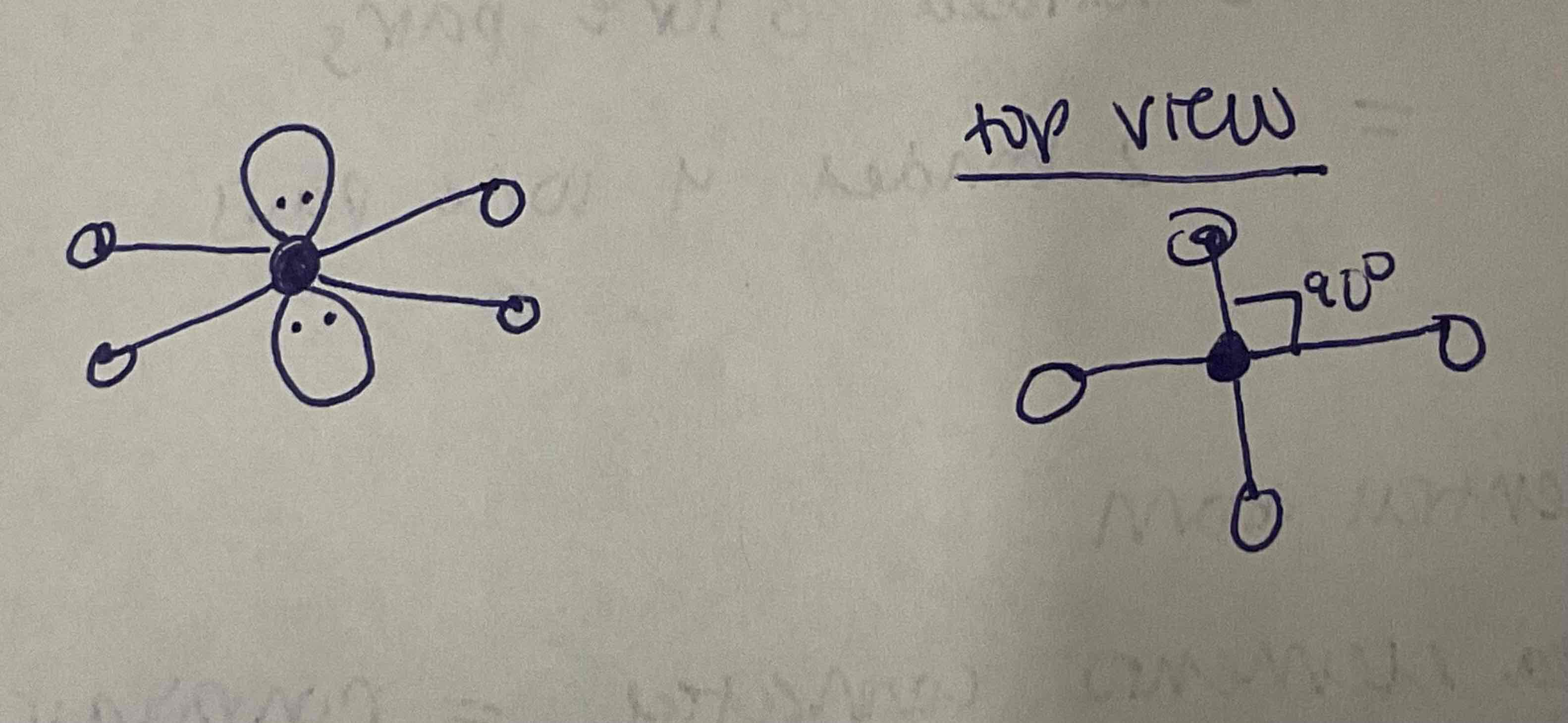
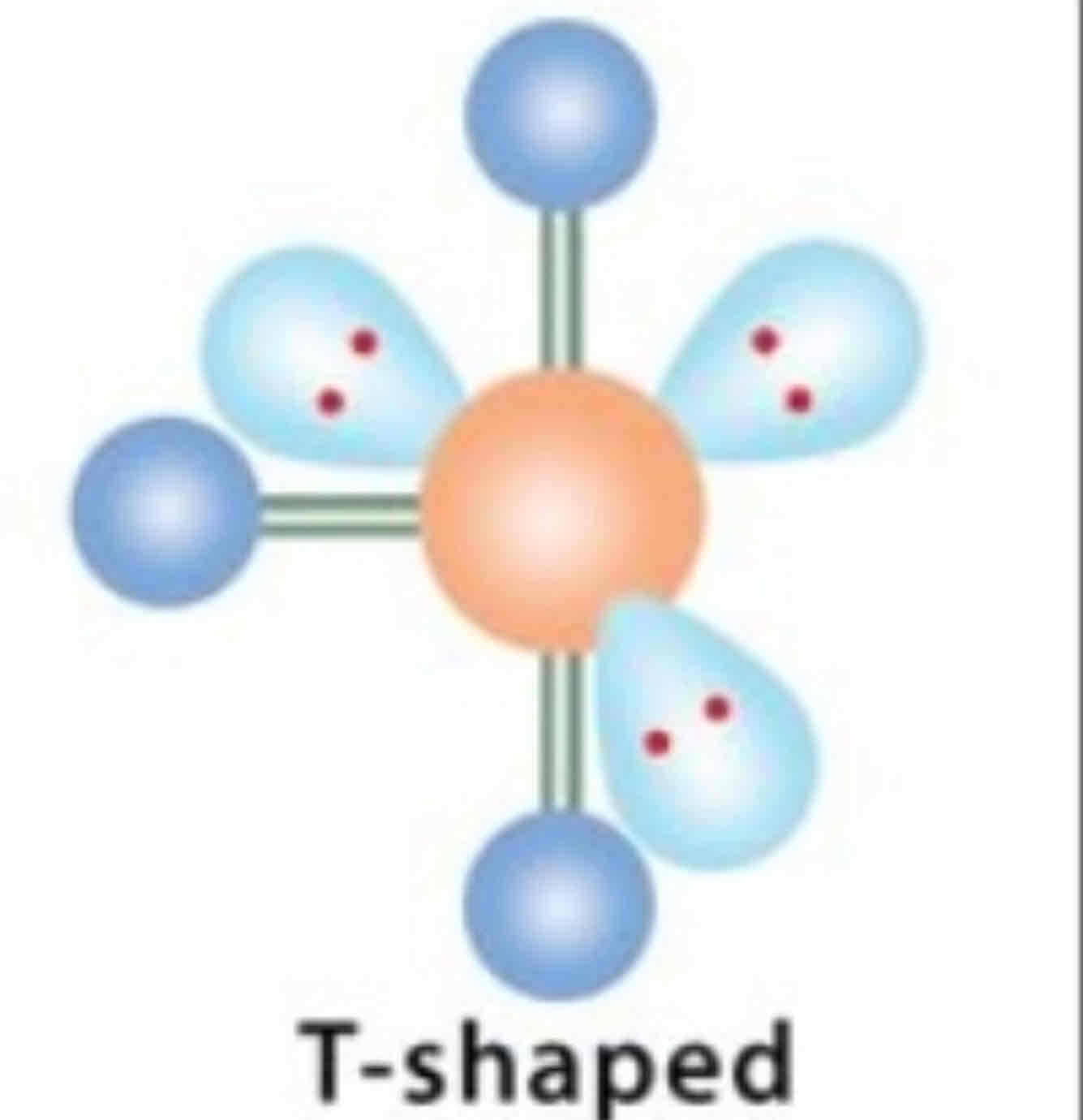
EDG and MG: 3 bonded pairs, 3 lone pairs
EDG: Octahedral
MG: T-shaped
EDG and MG: 2 bonded pairs, 4 lone pairs (not mentioned in class)
EDG: Octahedral
MG: Linear