StemUp: AQA A level Biology 3.1.4 Proteins
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
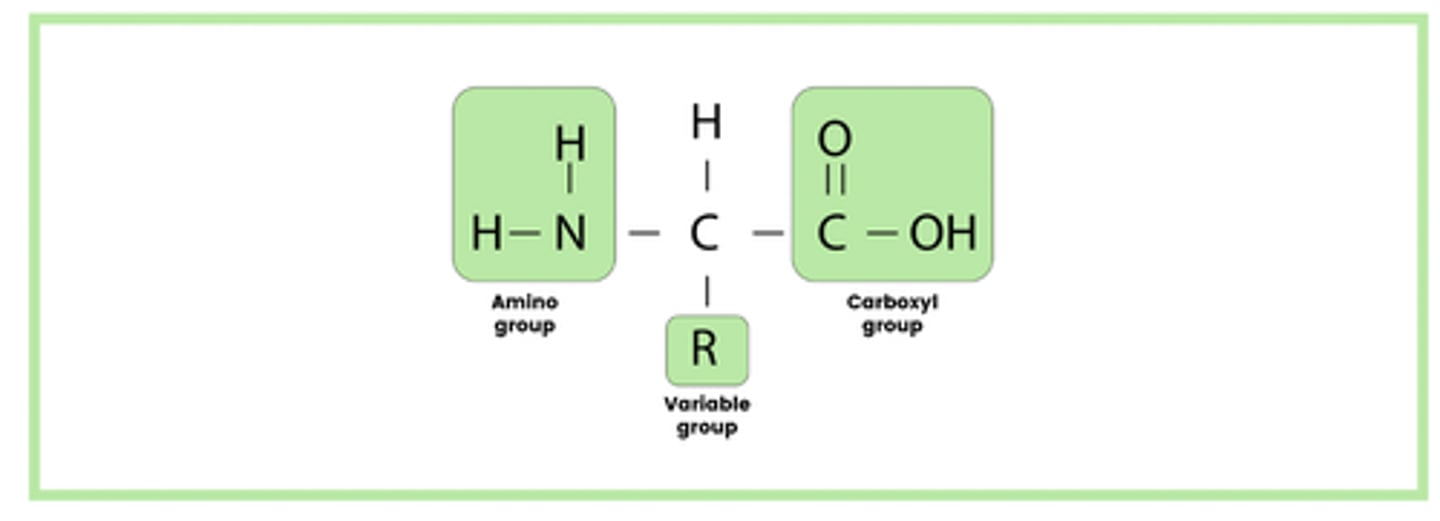
Draw the general structure of an amino acid (1)
- Amino group
- Carboxyl group
- Variable group
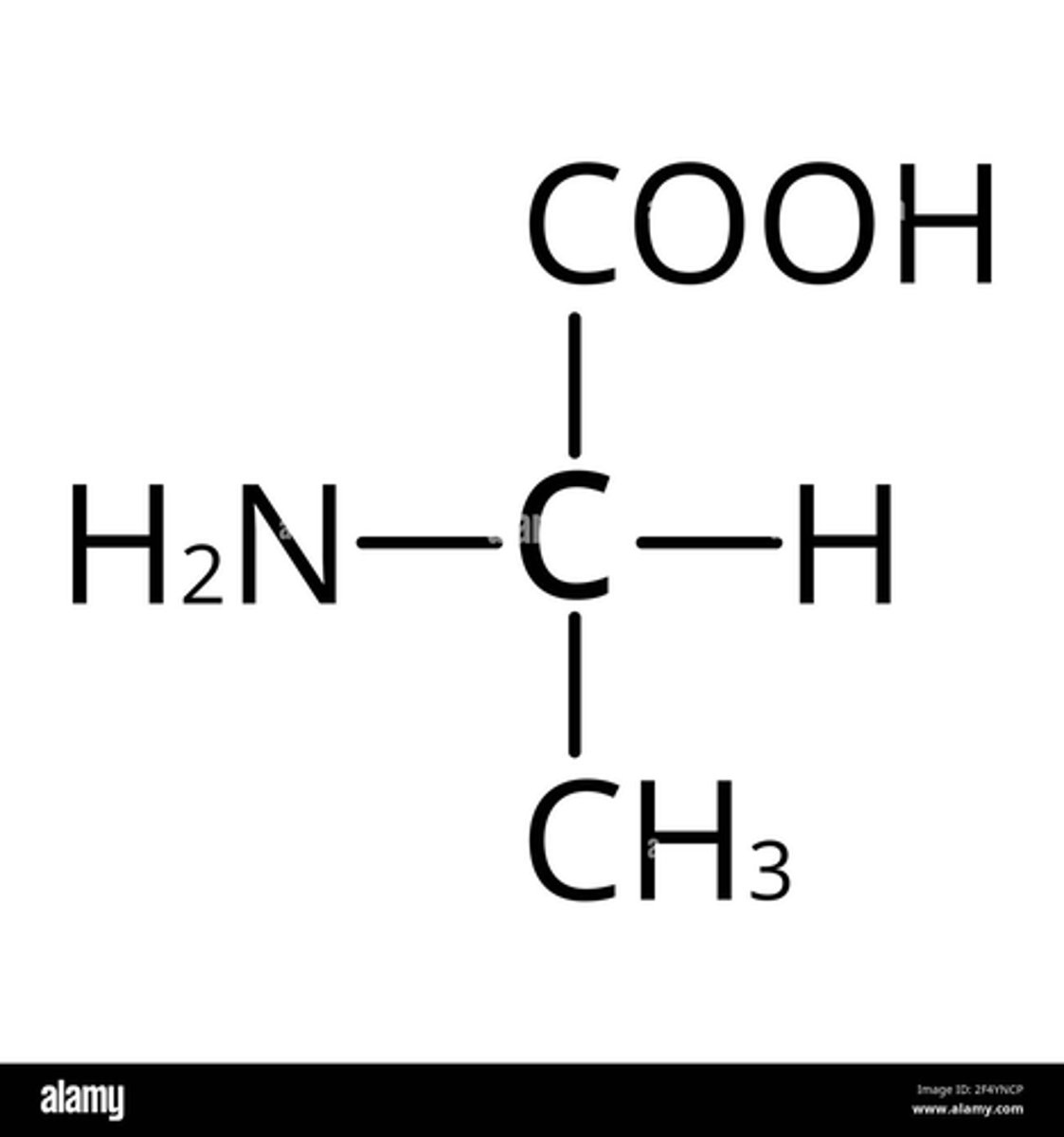
What do the twenty different amino acids that are common in all organisms differ in? (1)
Their R group
NOTE: you won't need to memorise them but you may need to apply your understanding of R groups in an exam so we have included an example
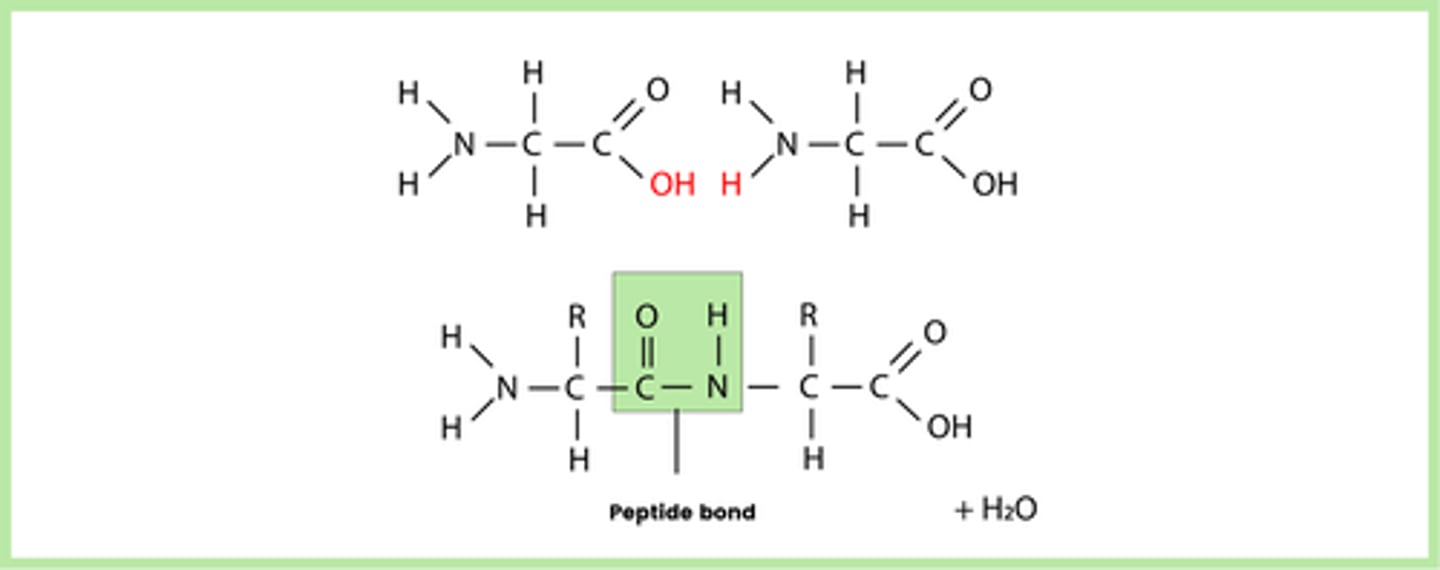
What does a condensation reaction between two amino acids form? (2)
- Peptide bond
- Represented by -CONH-
What do two amino acids joined together by a peptide bond form? (1)
Dipeptide
Describe how a peptide bond is formed between two amino acids to form a dipeptide (2)
- Condensation
- Between amine and carboxyl group
What do many amino acids joined together by a peptide bond form? (1)
Polypeptide
NOTE: A protein is a folded structure of a polypeptide, so 'protein' is still a valid answer. However, it is generally safer to refer to it as a 'polypeptide' in this context.
Draw a diagram that represents the formation of a peptide bond (1)
Note: AQA may ask you to draw a dipeptide (like this) or a short 3 amino acid chain polypeptide
What does a functional protein contain? (1)
One or more polypeptides
Describe what is meant by the the primary structure of a protein (2)
- The sequence of amino acids in a polypeptide chain
- Which determine the specific shape of a protein
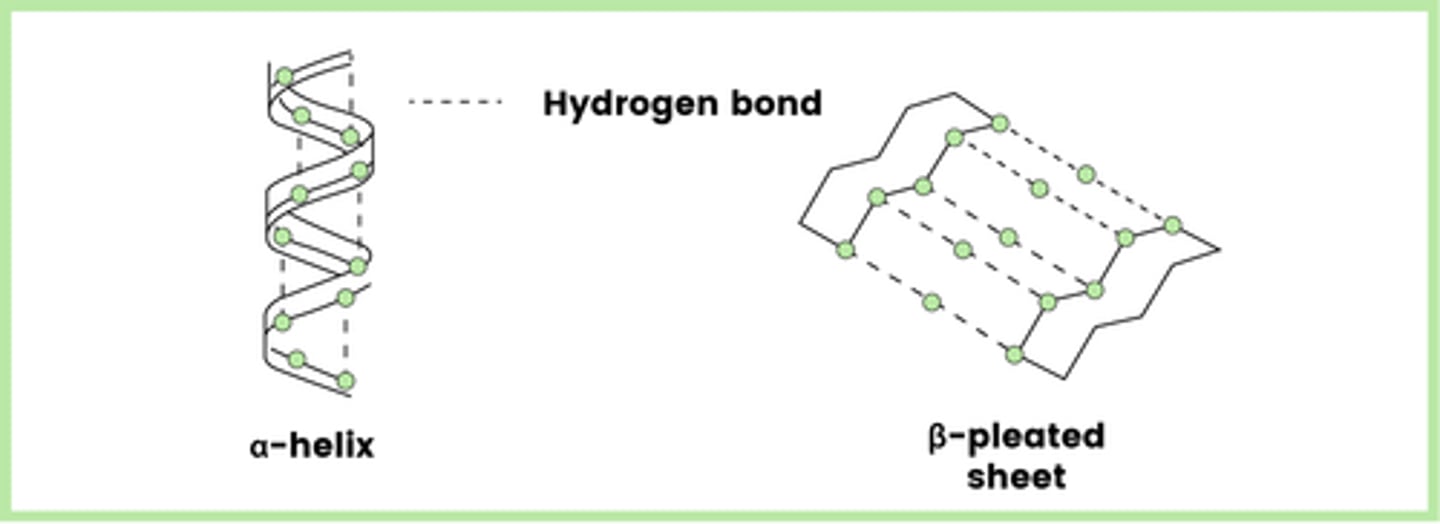
Describe how the secondary structure of a protein is formed (2)
- Folding or coiling of a polypeptide chain
- As a result of hydrogen bonds between amino acids
What are the two examples of secondary structures?
- α-helix
- β-pleated sheet
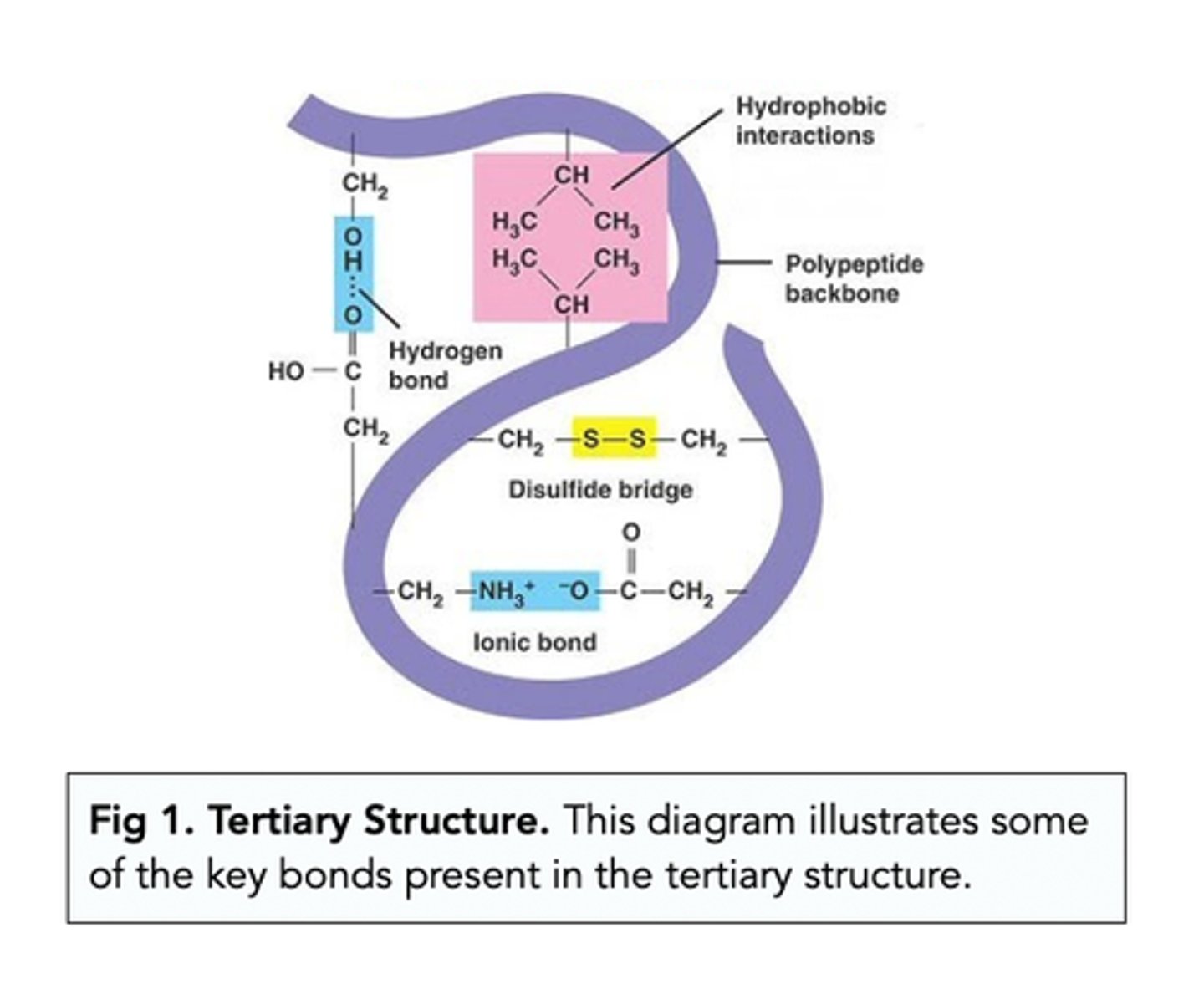
Describe how the tertiary structure of a protein is formed (2)
- Formed by the further folding and coiling of the secondary structure
- Due to hydrogen, ionic and disulfide bonds
What determines the formation of bonds in the tertiary structure of a protein? (2)
- Form in places determined by the primary structure
- Form between the R groups of amino acids
What are the two groups of proteins that you could be asked about?
- Globular
- Fibrous
NOTE: This has barely came up in past examination papers (as of 24-25) but its still useful to know and appreciate
What is the significance of fibrous proteins in terms of their tertiary structure? (1)
- They have little to no tertiary structure
- As they are long straight strands of amino acids with cross linkage hydrogen bonds
What are some examples of fibrous proteins? (3)
- Collagen
- Keratin
- Actin
What are some examples of proteins with a tertiary structure? (1)
Globular proteins e.g. haemoglobin, enzymes and antibodies
Draw a diagram of the tertiary structure of proteins, showing the structure of the bonds present (3)
What is the quaternary structure of a protein? (1)
Two or more polypeptide chains joined
What is an example of a quaternary structure? (2)
- Haemglobin
- As it contains four polypeptides joined together
Describe how you would carry out the test for proteins (3)
1. Add biuret reagent
2. Purple/lilac colour indicates that protein is present
3. If the solution remains blue, then no protein is present
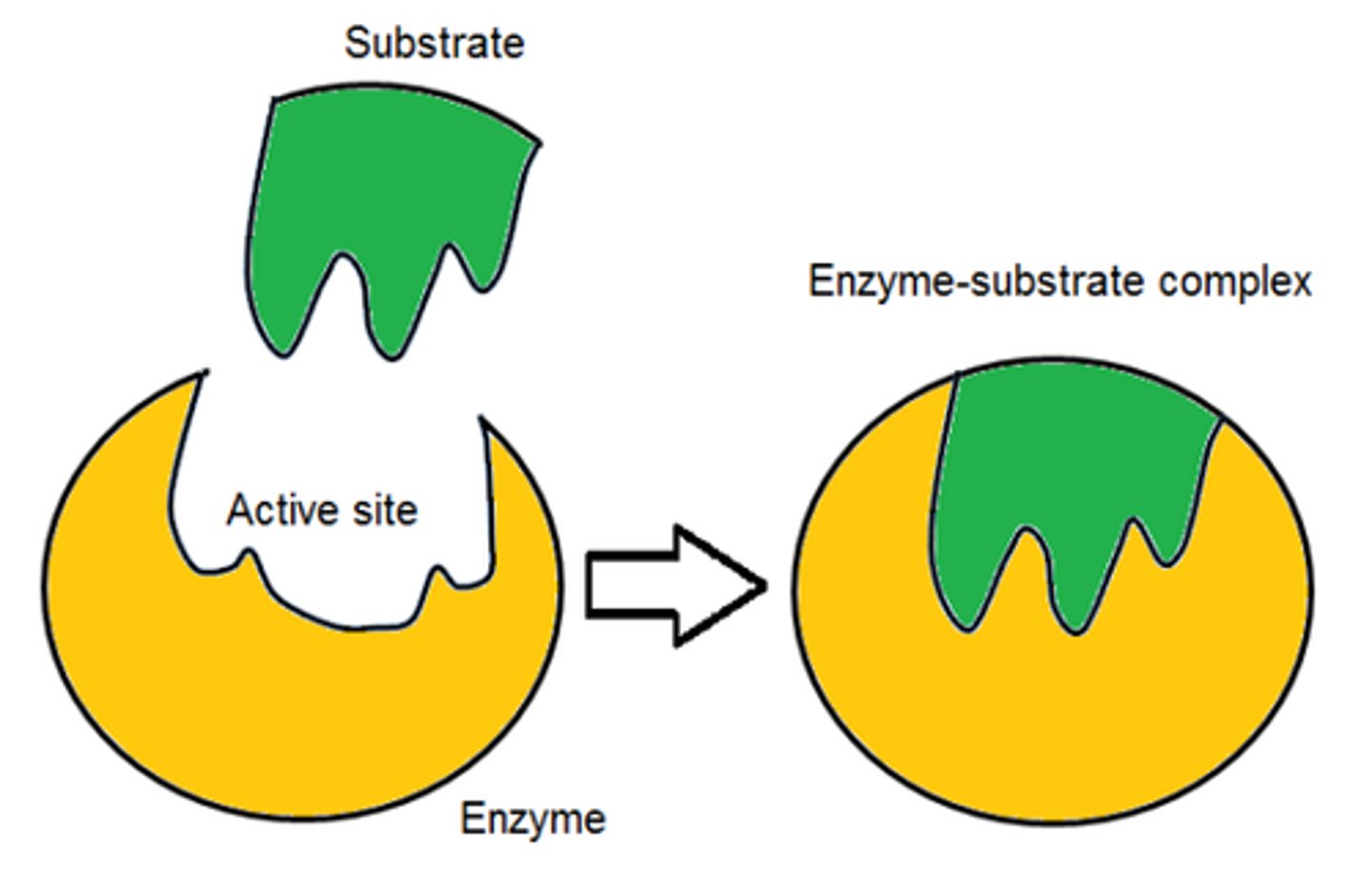
Describe how enzymes catalyse specific reactions (2)
- Lower the activation energy (the minimum amount of energy that is required for a reaction to take place)
- Through the formation of enzyme-substrate complexes (or E-S complexes)
NOTE: You wont necessarily be asked this in the exam but you will be expected to include these points in any question regarding substrates and enzymes fitting together
Explain why maltase can only catalyse the hydrolysis of maltose (3)
- Active site of maltase has a specific shape
- Only maltose can bind to it
- To form an E-S complex
Describe the lock and key hypothesis (1)
Only substrate molecules that are complementary in shape to the active site are able to form an E-S complex
NOTE: This is no longer in the specification but it can still come up in application questions about the induced fit model)
Describe the induced fit model of enzyme action (3)
1. Substrate binds to the active site of an enzyme
2. Active site changes shape slightly
3. So that it is now complementary to substate
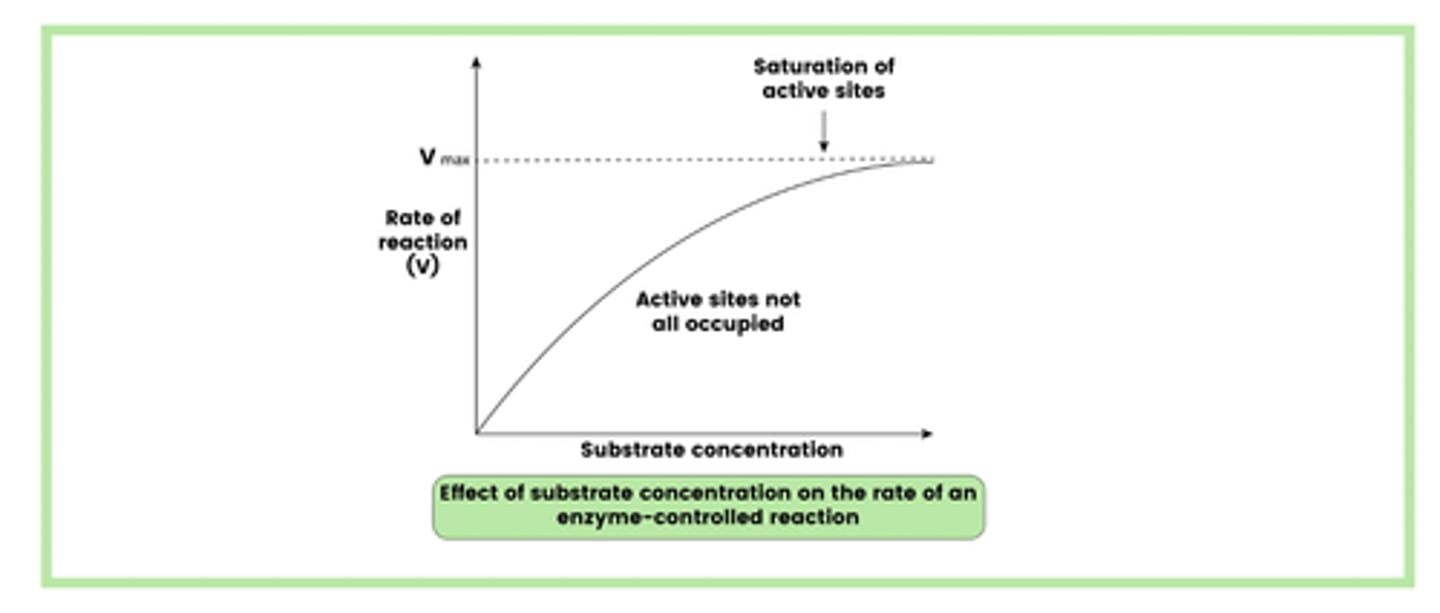
Describe the effect of substrate concentration on the rate of enzyme-controlled reactions (4)
1. The rate of reaction initially increases as collisions between substrate and enzyme molecules become more likely
2. The rate of reaction then levels out as the active sites of all enzymes become fully saturated by the substrate molecules
3. At this point, the rate of reaction is limited by enzyme concentration
4. The only method of increasing the rate of reaction is by adding more enzymes.
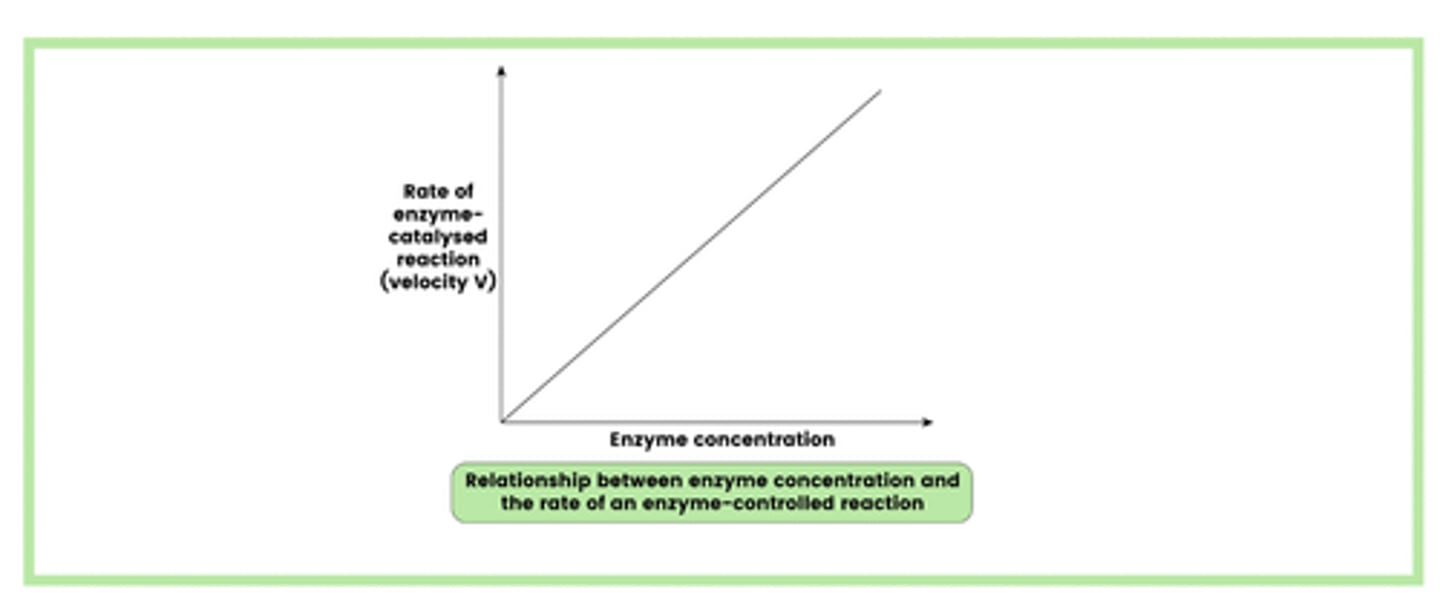
Describe the effect of enzyme concentration on the rate of enzyme-controlled reactions (4)
1. When the substrate concentration is in excess, an increase in enzyme concentration will increase the rate of reaction
2. This is because there are more enzyme molecules and, therefore, more active sites available
3. This increases the number of collisions between enzymes and substrates
4. Leading to the formation of more enzyme-substrate (E-S) complexes
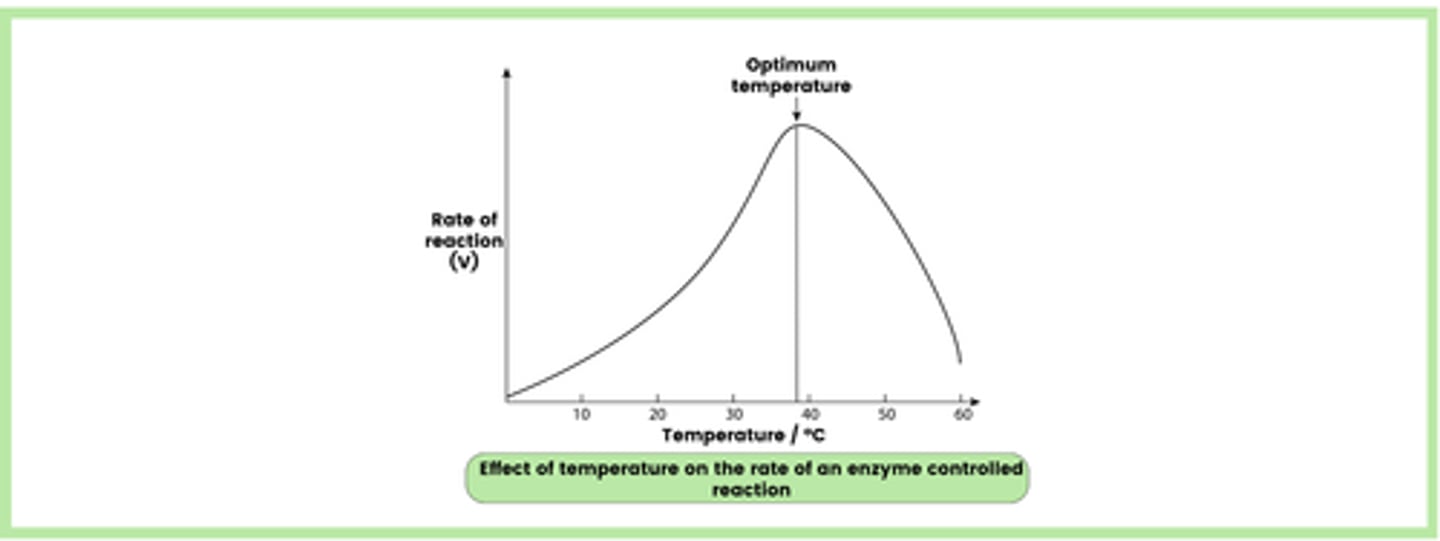
Describe the effect of temperature on the rate of enzyme-controlled reactions (6)
1. An increase in temperature provides molecules with more kinetic energy
2. This results in more collisions between active sites and substrate molecules, which leads to the formation of more enzyme-substrate (E-S) complexes
3. This increases the rate of reaction up to the optimum temperature, where the rate of reaction is at its maximum
4. Increasing the temperature above this point causes the tertiary structure of the enzyme to denature, as the hydrogen and ionic bonds are broken
5. The active site changes shape and is no longer complementary to the substrate, so fewer E-S complexes are formed
6. The rate of reaction decreases as the substrate has difficulty binding to the altered active site
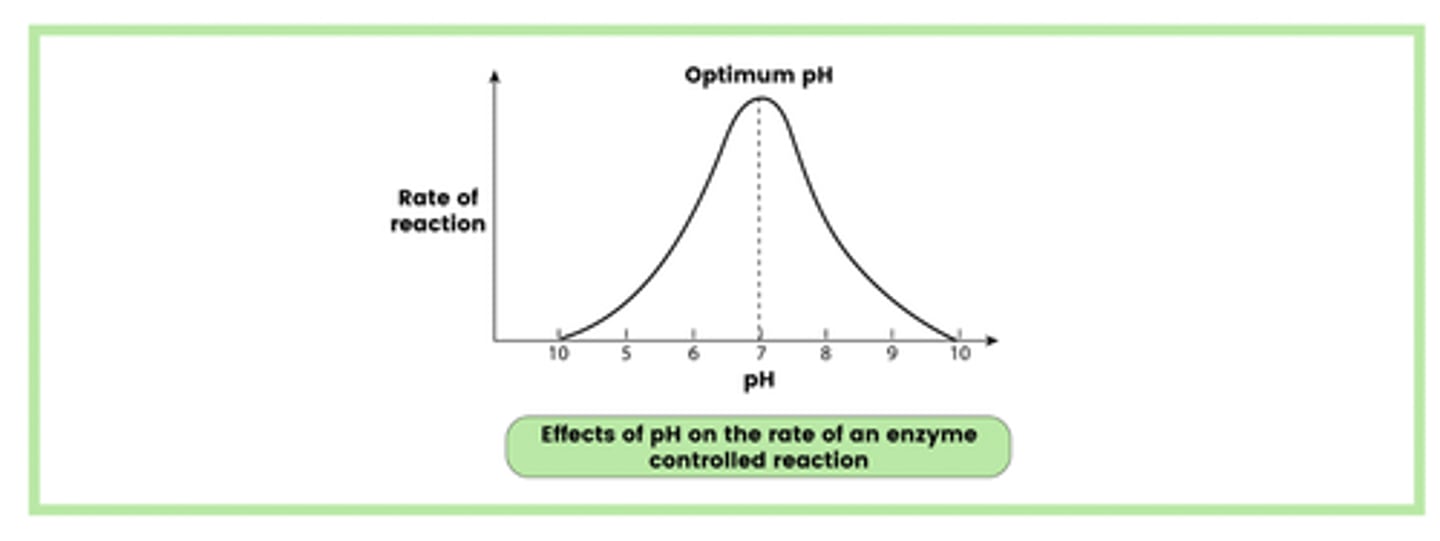
Describe the effect of pH on the rate of enzyme-controlled reactions (3)
1. A pH very different from the optimum causes denaturation
2. Changes in pH affect the ionic charges of the acidic and basic groups
3. Hydrogen and ionic bonds are broken, altering the tertiary structure and, therefore, the shape of the active site.
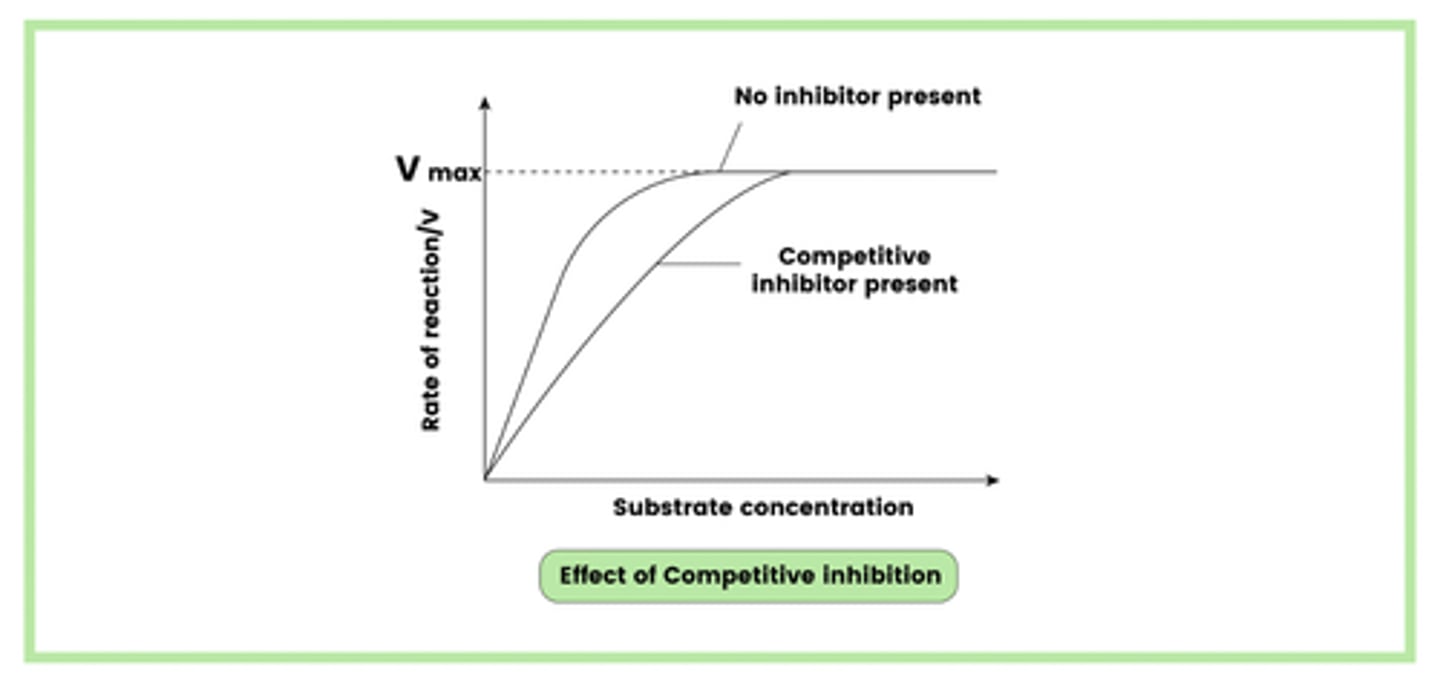
Describe how a competitive inhibitor can reduce the rate of an enzyme-controlled reaction (3)
1. Inhibitor has a similar structure to the substrate and competes with it for the attachment to the active site
2. Rate of reaction is reduced as the substrate cannot bind to an occupied active site
3. Competitive inhibition can be reduced by the addition of more substrate
Draw a diagram to illustrate an enzyme-controlled reaction in the presence of a competitive inhibitor (1)
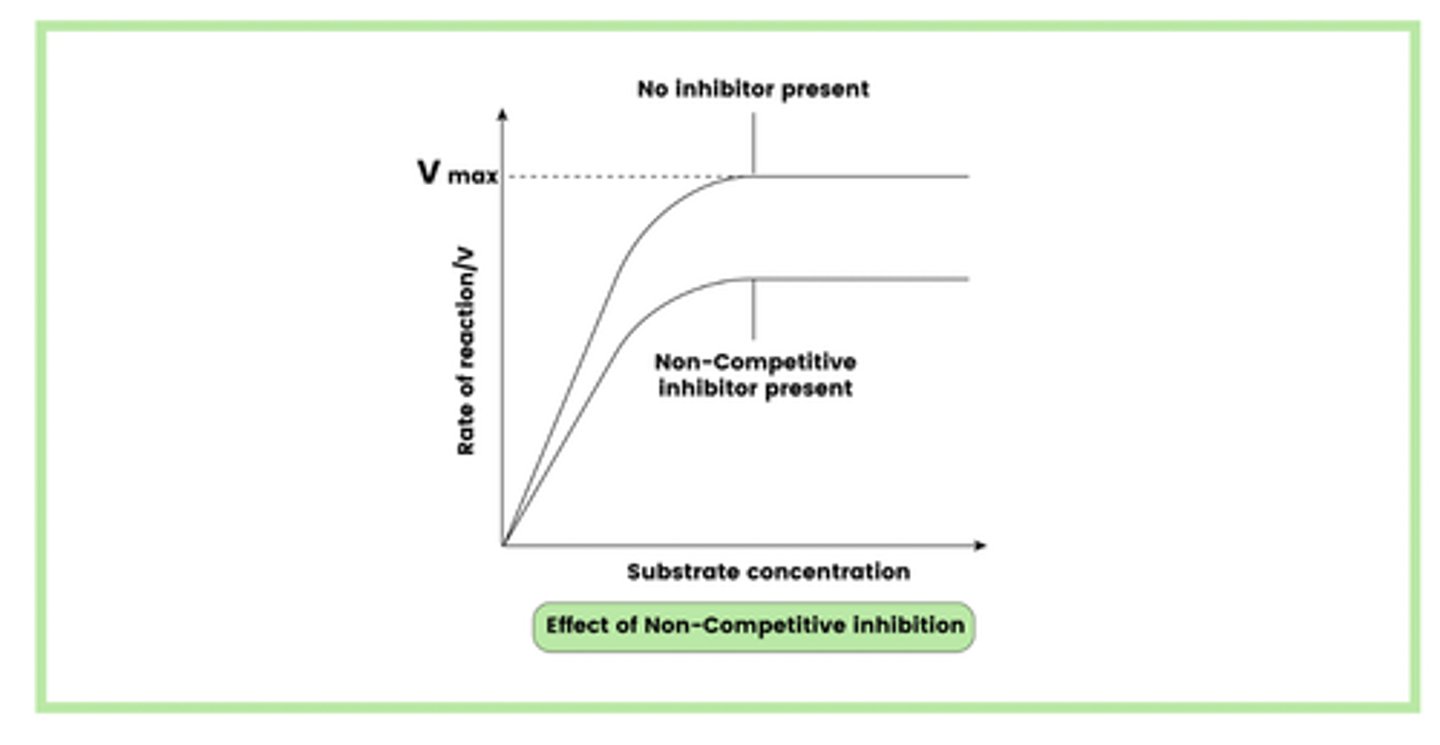
Describe how a non-competitive inhibitor can reduce the rate of an enzyme-controlled reaction (4)
1. Inhibitor attaches to the enzyme at a site other than the active site (allosteric site)
2. This changes the shape of the active site
3. Which means the active site is no longer complementary to substrate
4. So less E-S complexes form
Draw a diagram to illustrate an enzyme-controlled reaction in the presence of a non-competitive inhibitor (1)