Disproportionation and water treatment
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
disproportionation reactions
When a single element simultaneously is oxidised and reduced
Making bleach
Mix chlorine gas with cold, dilute aqueous sodium hydroxide → sodium dichlorate
Chlorine is both oxidised and reduced

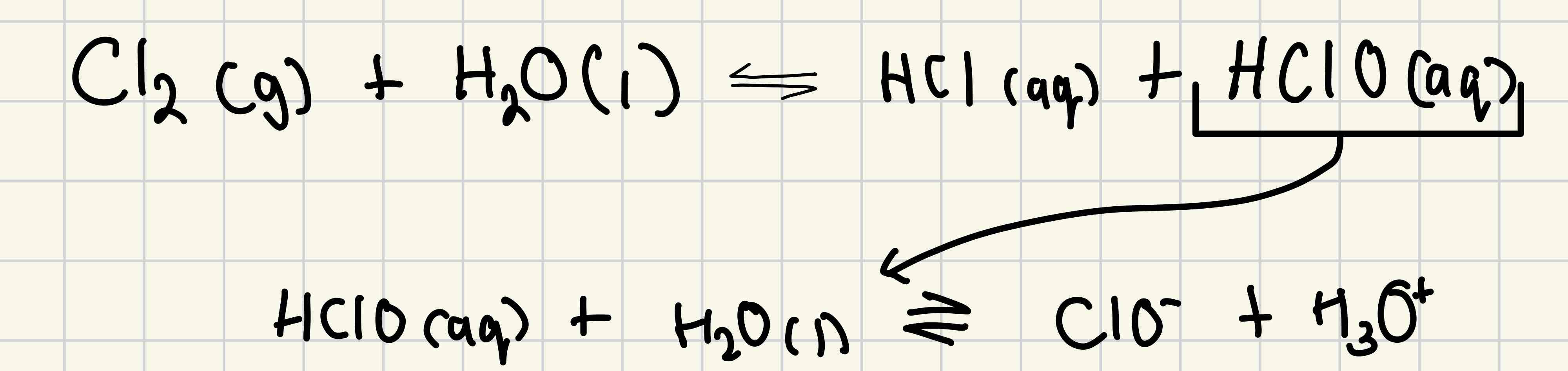
Chlorine and water
Mix chlorine with water = disproportionation reaction
= Mixture of hydrochloric acid and chloric (I) acid
Aqueous chloric(I) acid ionises to make chlorate (I) ions
Water treatment
Chlorate (I) ions kill bacteria, add to water → safe to drink / swim in
Prevents algae growing
Eliminates bad tastes / smells
Removes discolouration by organic compounds
Risks of using chlorine for water treatment
Chlorine gas is toxic
Harmful to breathe in - irritates respiratory system
liquid chlorine → cause chemical burns to skin or eyes
Chlorine + organic substance ( from water from decomposition of plants )= chlorinated hydrocarbons → cancer but risk is lower than risk from unclean water
Seen as mass medication → forced, no choice to not have chlorinated water
Alternatives to chlorine
Ozone → oxidising agent = kills micro organisms - expensive, short half life so doesn't last long
UV lights → kills microorganisms by damaging DNA, ineffective in cloudy water and doesn't last long