02 Structure of Materials
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
What is the atomic weight?
The weighted average of the atomic masses of the atom’s naturally occurring isotopes
What is the heaviest natural occuring element?
Uranium (92)
What represents the atomic number?
The number of protons in nucleus
What are isotopes?
Species with the same number of protons but differing number of neutrons
Same name, different mass
What happens with the energy of electrons?
Is quantified and defines the electron’s position in an energy state (Bohr) or in a shell (Schrödinger)
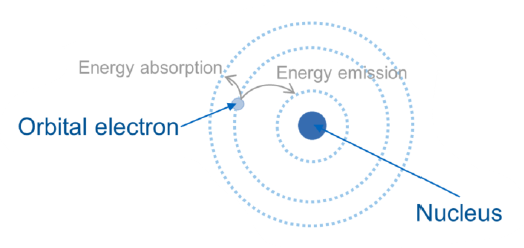
What is the implication of energy absorption of an electron?
Jump to a higher energy level (more outer orbitals)
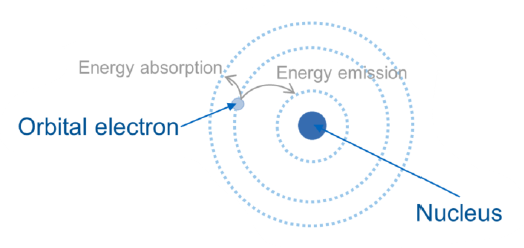
What is the implication of energy emission of an electron?
Jump to a lower energy level (more inner orbitals)
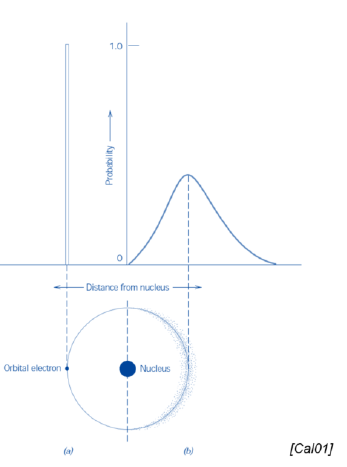
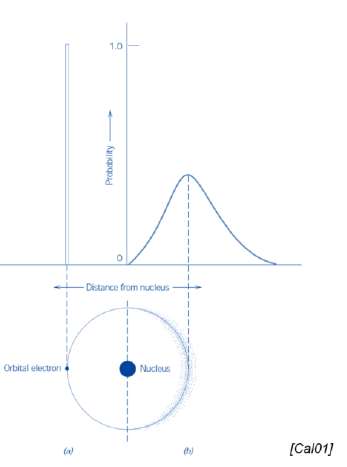
What is the main difference between the Bohr and the wave-mechanical atomic models?
Bohr: discrete energy levels (and electrons)
Wave-mechanical: probabilistic distribution of energy levels (and electrons)
What are quantum numbers used for?
To specify size, shape and spatial orientation of an electron’s probability density
What do shells describe?
Distance of an electron from the nucleus (position)
How are shells specified?
By quantum number n = 1, 2, 3, 4, 5 …
Letters K, L, M, N, O related to each numbers (respectively).
What do subshells describe?
Shape of the electron subshell
How are subshells specified?
s, p, d, or f (lowercase)
What do the other two quantum numbers describe?
Spatial orientation of the electron orbital angular momentum
Magnetic spin of an electron
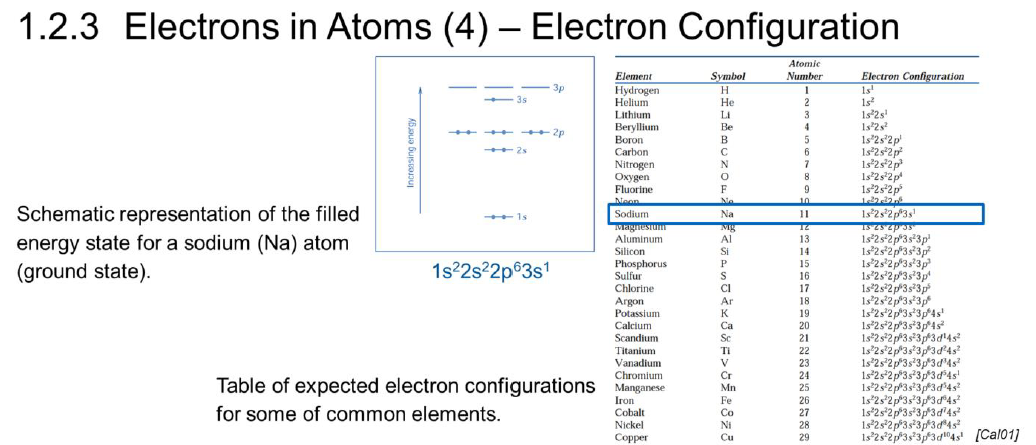
What is the electron configuration?
How electrons are distributed within the orbitals of an atom
Pauli Exclusion Principle
Each electron state can hold no more than two electrons, which must have opposite spins
How many electrons can each subshell acommodate?
s: 2
p: 6
d: 10
f: 14
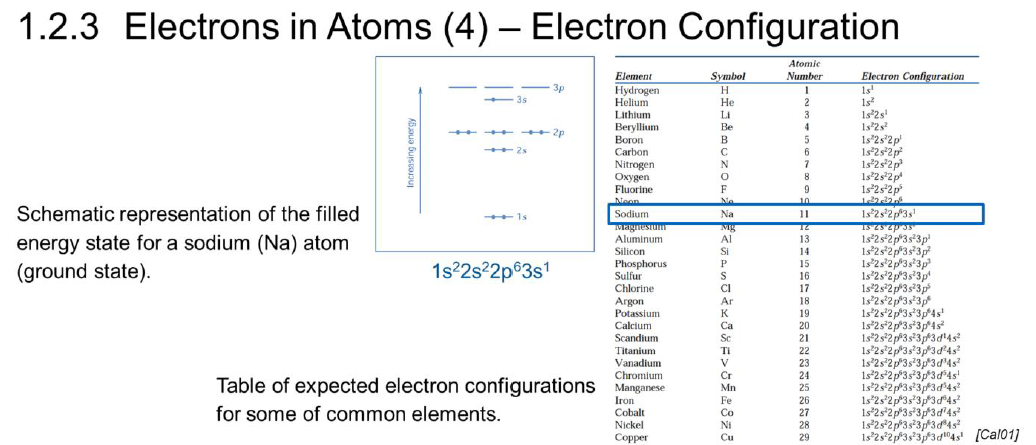
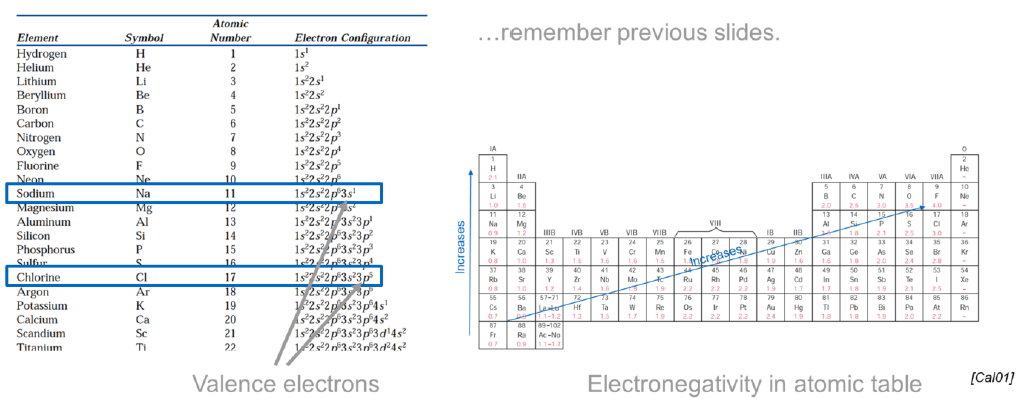
What are valence electrons?
Electrons on the outermost filled shell
Participants of bonding
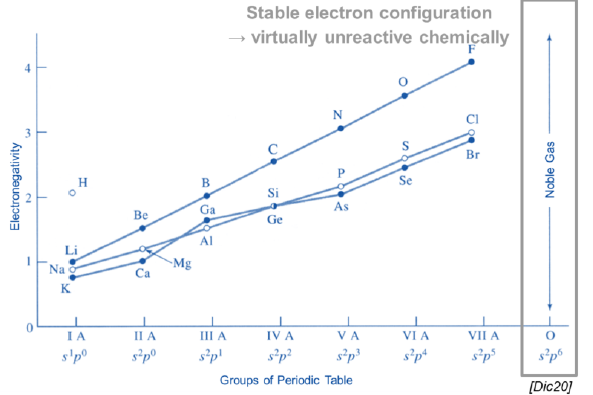
What is the electronegativity? What is its trend behavior?
The attraction of the atomic nucleus to an additional electron
Increses moving from left to right and from bottom to top in the periodic table
The higher the electronegativity, the proner the element is to accept electrons (the more reactive)
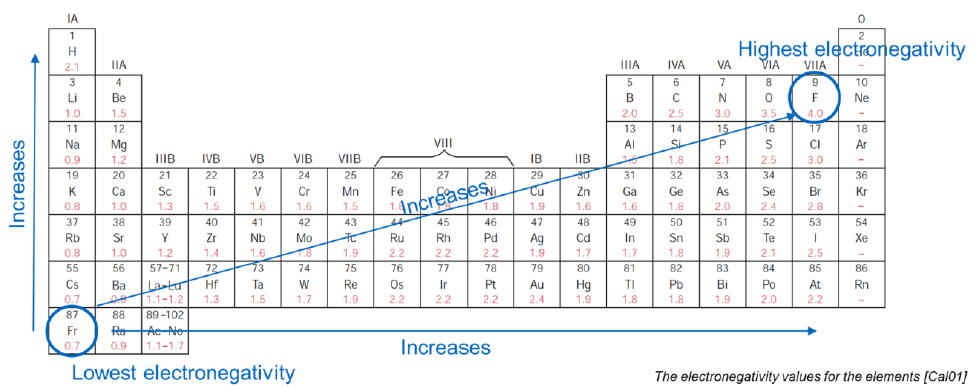
What is the most electronegative element? What is the less?
Most: Fluorine
Less: Francium
Which kinds of bonds are there? (4)
Ionic
Covalent
Metallic
Van der Waals (secondary, i.e. between atoms of different molecules)
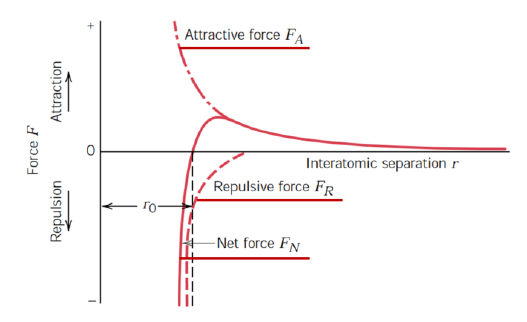
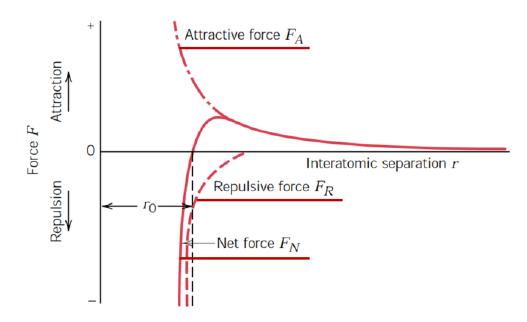
Describe the interaction between two atoms in the sphere-spring model
Spring between atoms
Magnitude of force as a function of the interatomic distance
Attraction forces with a + sign (FA) due to atomic bond
Repulsive forces with a - sign (FR) due to electromagnetic charges
Net force FN = FA + FR
Both attractive and repulsive force decay with the distance
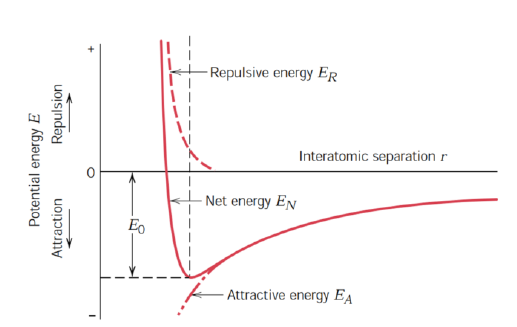
How is the energy of a bond calculated?
Integral of FN wrt. interatomic distance r
Positive for repulsion
Negative for attraction
Equilibrium achieved for FN = 0 and minimum energy
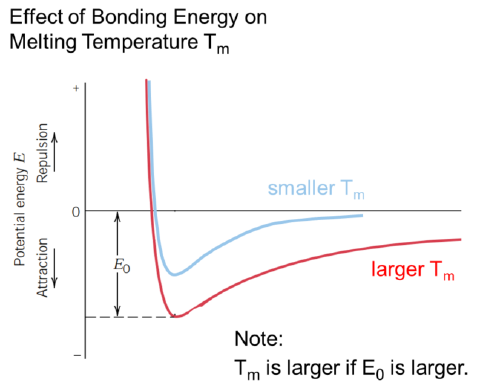
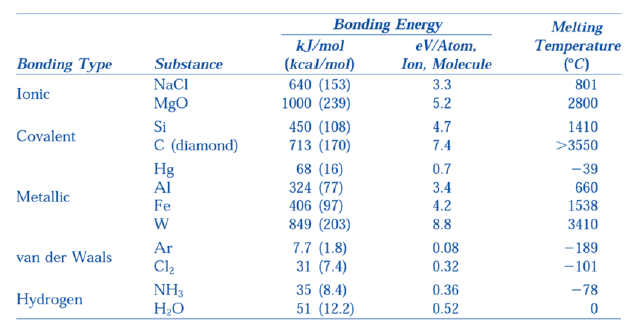
How is the bonding energy related to the melting temperature?
The larger the bonding energy, the larger the melting temperature
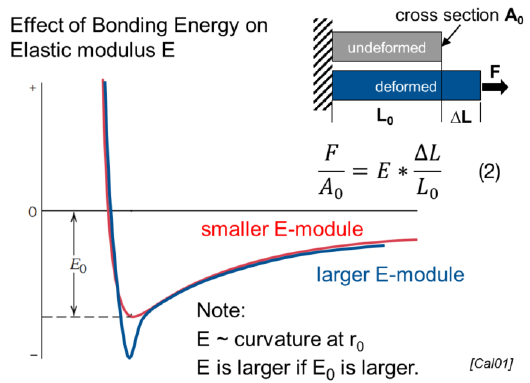
How is the elastic modulus E related to the bonding energy?
The larger the bonding energy, the larger the E modulus
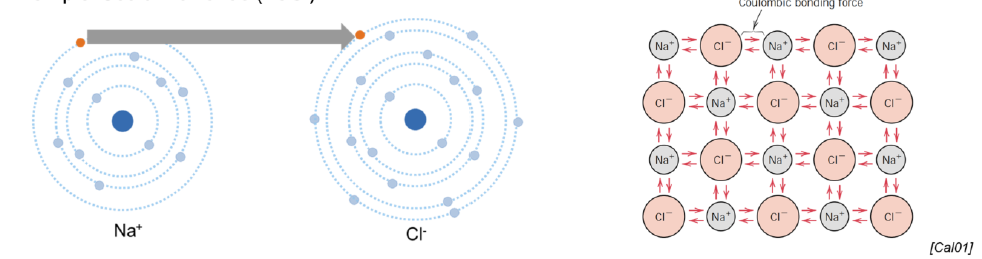
Describe how ionic bonds are formed
More electronegative atom attracts electrostatically the valence electron(s) of the less electronegative atom
For differences in electronegativity > 1.8
Ex: ionic salts, metallic-non metallic bonds
What are the main characteristics of ionic compounds?
Create regular lattice structures (crystalline structures)
Hard and brittle
Electrically and thermally insulative
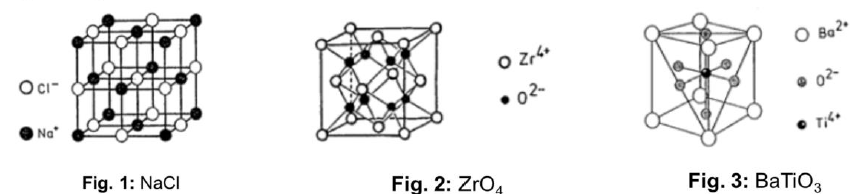
Describe how are covalent bonds are formed
Each of the two atoms share at least one valence electron to the bond
Stable electron configuration assumed
Directional bonds, belonging only to sharing atoms
For an atom with N’ valence electrons, it can bond with at most 8-N’ atoms
For differences in electronegativity < 1.7, both with electronegativities larger than 1.5
What are the main characteristics of ionic compounds?
Usually stable
Orientation and distance of atoms fix defined for covalent bonds
Ex: crystalline Ge, Si, C; methan; water; polymers
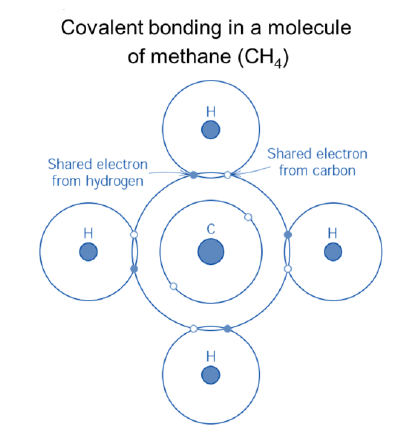
Describe how are covalent bonds are formed
Sea/cloud of valence electrons (electron gas)
Positive charged atom cores forming lattice structures (cubic space centered, cubic face centered, hexagonal) depending on the type of atoms
What are the main characteristics of ionic compounds?
Good electrical and thermal conductivities
Good deformability between lattice planes
Chemical reactivity
(Generally) weaker than covalent and ionic bond (lower hardness compared to ceramics)
How are Van der Waals bonds formed? What are their characteristics?
Result from coulombic attraction between positive end of a dipole and the negative end of another (dipole usually formed by covalent bonds)
Could be formed by symmetrical or induced dipoles
Keep together already formed molecules or chains
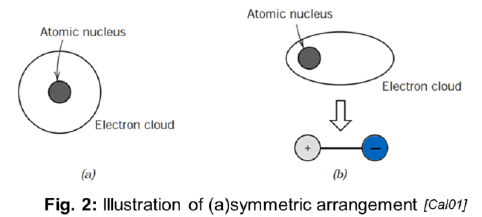
SQ: Name the two atomic models cited, and note the difference between them
Bohr atomic model and wave-mechanical atomic model
Difference:
Bohr: asummes electrons located at well defined orbits
Wave-mechanical: assumes electrons whose position is not well-defined in atomic orbitals. Position as a probability distribution.
SQ: Describe the important quantum-mechanical principle that relates to electron energies
Energy of electrons is quantized in discrete values
An energy change (absorption or emission) leads to a change of orbital (outer for absorption, lower for emission)
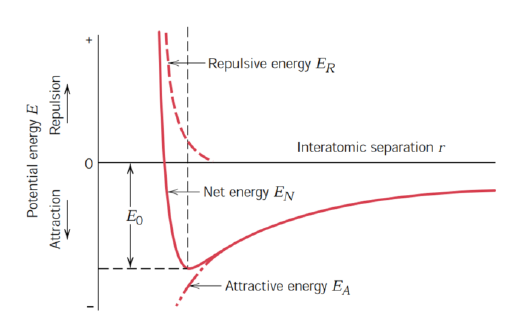
SQ: Schematically plot attractive, repulsive, and net energies versus interatomic separation for two atoms or ions
Everything goes to 0 with an increase of r
Net energy is a sum of the attractive energy and the repulsive energy
Attractive energy with - sign
Repulsive energy with + sign
SQ: Briefly describe ionic, covalent, metallic, and secondary (van der Waals or hydrogen) bonds
Ionic: stolen atom for ΔEn (difference of electronegativity) > 1.8. Leads to strong, brittle, insulative, crystalline materials
Covalent: shared electrons for ΔEn < 1.7. High stability (hardness) and fixed spatial position
Metallic: sea/cloud of electrons with positive charged cores forming lattice structures. Form conductive, ductile, reactive compounds
Van der Waals: coulombic attraction between symmetric or induced dipoles of already formed molecules/ chains (secondary bond)
SQ: Note what materials exhibit each of these bonding types
Ionic: NaCl, MgO, etc.
Covalent: Ge, Si, C
Metallic: iron, mercury, wolfram, some alloys
Van der Waals: polymers