Molecular Modeling
1/233
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
234 Terms
What is a “polarization” basis set?
adding the next function up (d-function to p-block)
How does the use of polarization basis set allow calculations to reflect actual electron behavior in molecules and ions?
move the electrons off the nucleus which is more accurate and can improve the molecular models
what are “diffuse functions”
made up functions that describe the electrons far from the nucleus that the gaussians can’t accurately describe
Why are diffuse functions added to basis sets?
added to improve electron descriptions for more accurate calculations
How is a basis set containing diffuse functions denoted?
with a + plus
What is the difference between * and **
one indicates the addition of polarization to basis set only to heavy atoms while the other indicates polarization is added to all atoms
what does 6-311+G** mean
split valence basis set with 3 orbitals. 3 contracted, 1 diffuse, and 1 intermediate. 6 guassians used for core electrons. + is adding diffuse functions then both ** indicates polarization to all atoms
How are molecular orbitals obtained in quantum mechanics calculations?
by combining atomic orbitals to create the molecular orbitals
what is a “basis set”?
electron description in a wave function
What are slater Type orbitals
orbitals produced from atomic functions obtained from solution of 1 electron shrodinger equation (s,p,d)
describe two ways that the Gaussian function differs from Slater Type Orbitals
very far from the nucleus and very near the nucleus
given these differences where might the use of Gaussian functions introduce error in quantum calculations?
for core electrons and for anions and etc.
what does STO-3G stand?
slater type orbitals -3 gaussians
Describe the STO-3G
three gaussian functions to describe slater type orbitals
The STO-3G basis set is a “minimal” basis set. What does this mean?
using only as many functions as needed to represent valence and core shells
what are the two major shortcomings of a minimal basis set?
sum of minimal basis set in a sphere. sum of minimal basis set is nuclear-centered
what is a “split-valence” basis set?
splitting every valence shell in 2
what is meant by “contracted” or a “diffuse” orbital
a condensed orbital or a spread orbital
How does the split-valence basis set address one of the shortcomings of a minimal basis set?
gets rid of the spherical symmetry
What magnetic property arises when a molecule or ion has unpaired electrons?
magnetic susceptibility
Does the electron dot structure of oxygen predict that it will be paramagnetic of diamagnetic?
diamagnetic
What are the basic tenets of the molecular orbital model?
Adjacent atomic orbitals combine and make MOs. MOs only form from AO with approximately similar energies and symmetry. there are bonding, antibonding, and non-bonding MO.
What is a MO diagram?
a diagram that shows how e- are distributed in orbitals.
what is a “bonding” MO? How is it characterized?
attractive form between nuclei. characterized by lower energy than the AOs from which the electrons come from?
what is an “anti-bonding” MO? how is it characterized?
repulsive force between nuclei. characterized by higher energy then the AOs from which the electrons came from. nodal plane.
what is a nonbonding molecular orbital? how is it characterized
like lone pairs. characterized by being comparable energies to the original AOs the electrons were originally pulled from. centered on an atom.
according to MO model, why would H2 form but He2 would not
the first is lower in energy than the original atoms alone and the second is higher in energy than the original atoms alone.
How would a MO diagram demonstrate a paramagnetic molecule?
by having at least one MO with an unpaired electron
Experimentally, how do we know that the ordering of the MOs is different for N2 than O2
photoelectron spectroscopy
What property of O2 does the MO model get right that the other models fail to predict
the proper magnetism
What is a LUMO
the lowest unoccupied molecular orbital
what information does the LUMO provide?
what spot can accept an electron. ability to accept an electron
What is a HOMO
highest occupied molecular orbital
What information does the HOMO provide?
where donated electrons come from
What would happen to the bon length if an electron were removed from an antibonding orbital forming a cation.
would get shorter
what would happen to the bond length if an electron were removed from a bonding orbital forming a cation?
would get longer
What are conformations?
2 different structures for the same molecule
will two equilibrium geometry calculations always result in the same structure
no, they could have different starting structures with different minimums
What is the difference between a global minimum and a local minimum
the first is an overall minimum. the second is the minimum in that area
What is a single point energy calculation
you build and calculate without changing the geometry
why does the time taken to perform a single point energy calculation scale more linearly than a geometry optimization as the number of atoms
for the first, it only matters that you’re adding an atom so it is linear. The second adds multiple degrees of freedom per atom which is why its not linear
What is symmetry?
when there is a line of symmetry on a molecule’s connectivity
why does invoking symmetry decrease the time it takes for a calculation to finish?
it forces certain points to be the same
What is a geometric constraint?
a constraint that usually applies that a certain measure should be applied to another as well
When would a geometric constraint be used?
when you just want to calculate what the molecule you’ve drawn’s energy is
What is a potential energy surface?
graph of potential energy of a molecule at different lengths/angles/dihedrals
what is meant by geometric degrees of freedom
length, angle, or dihedral angle
What is an energy gradient?
the slope of the line tangent to the potential energy surface.
What determines the magnitude of the energy gradient?
how fast the potential energy changes, slope
How does a modeling program locate an energy minimum?
taking small steps to find lower energy until no possible step brings energy down
what is meant by “convergence”
converging on a minimum single, stable structure
Why do the calculations for some species have difficulty converging?
can happen by characterizing unstable molecules with shallow, broad potential energy surfaces
What can be done to improve the chances of convergence of a high-energy species
taking bigger steps
why does the time it takes to perform an equilibrium geometry optimization scale exponentially as the number of atoms increases?
exponentially increasing energy and degrees of freedom
what are the typical parameters used in molecular mechanics?
length at equilibrium, bond angle at equilibrium, dihedral angle at equilibrium, spring constant, and size of molecules
What is the primary molecular mechanics model used in spartan
SYBL and MMFF (mainly MMFF)
Why SYBL and MMFF molecular mechanics methods yield different results for the energy of a molecule
they have different parameters and assumptions. they work better on different types of molecules
if strain energy is always positive, how can the total energy difference obtained from a molecular mechanics calculation be negative?
electrostatics can cause this. can be negative if there are opposite charges attracting and causing “strain”
what limitations are there for comparing energies obtained from molecular mechanics
to compare energies, have to typically be same bonds and same bond types
why is it important that each bond type be defined for any structure on which a molecular mechanics calculation is performed
you have to know the bond order to compare the the bond to known values
why is molecular mechanics used to describe the energies of proteins?
because we can. can’t use some other models because of protein molecule size.
parameters such as bond lengths, bond angles, and vibrational frequiencies seem to be transferable between similar types of molecules. for example, such that most C=C bonds are similar. how does this observation lead to the concept of molecular mechanics?
parameters help set a standard for these and molecular mechanics can then use these but also somewhat predict deviations
how are the bonds and angles described in molecular mechanics
they are described as springs
what is a “strain energy”
energy it takes to deviate from equilibrium position
how is the energy from molecular mechanics determined?
hooke’s law
given that molecular mechanics assumes bonds to be atoms held together by springs, why does this method work reasonably well
because at equilibrium bonds behave exactly like springs
what is the force constant in molecular mechanics
parameter the represents bond stiffness in a chemical bond. essentially how much force per unit length taken to stretch or compress
how are force constants determined experimentally
by IR. Using different methods then analyzing the vibrational frequency
why is molecular mechanics sometimes called “atoms on springs”
treating the bonds like springs. its the basis of the method
what is “parameterization”
you set different parameters for the different methods. basis of models are determined by parameters
do equivalent electron dot structures contribute equally to the observed structure
yes
do non-equivalent electron dot structures contribute equally to the observed structure?
no
Why can S atoms have more than 8 electrons
they have access to the d orbitals
for elements beyond the second period, how can formal charge be reduced
using the d valence shell
what must be remembered about the connectivity of the atoms in oxy-acids
typically connected in layers
what is a peroxide bond
O single bonded to O
why are peroxide bonds generally uncommon in naturally occurring compounds
they are unstable and highly reductive
which structure for sulfuric acid best describes the molecule and why
a sulfuric acid structure with the 0 formal charge as S has access to the d valence shell so it can have the extra connectivity
what is an electron dot structure
demonstrating atomic connectivity using letter abbreviations for atoms and dots for electrons
what is the physical basis of the octet rule.
if an atom is in p-block, the typical valence shell is usually 8 electrons so an “octet” fills its valence shell
what is a bond pair of electrons
a line aka electrons shared behind two bonded atoms
what are lone pair electrons
valence electrons that are not shared between atoms
how can formal charge be used to predict the relative stability of two species
typically species without formal charge are most stable or whichever has the least formal charge
what is bond order
measure of multiplicity of a covalent bond between two atoms. single bond= 1st order. double bond=2nd order. triple bond=3rd order
what is the relationship between bond order, bond length, and bond strength
higher order means stronger and shorter
what is the process of using two or more electron dot structures to represent a single molecule called
resonance
what is the difference between equivalent and non-equivalent electron dot strucutre
equivalent: each contribute equally to actual connectivity of a molecule. non-equivalent: don’t contribute equally, typically electrons in very different spots
what determines the chemical properties of an atom, ion, molecule
mainly the valence shell but overall the electron configuration dictates the chemical behavior
what is meant by ab initio
“from beginning”, starting with nuclei and electrons
who was Erwin Schrodinger and how did he describe an atom
He was an austrian physicist who described an atom. he had the model of an atom that described electrons more as waves
what is a wave function
a mathematical function that relates the location of an electron at a given point in space to the amplitude of its wave
how is a wavefunction related to an electron orbital
An orbital is a region of space where there is a high probability of finding an electron the wavefunction provides the mathematical description of that region
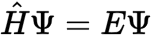
The Schrodinger equation is given by (image). what does the H mean
its a Hamiltonian operator, its a mathematical function
Who was Henri Poincare?
french math person. says you can't solve the three body problem. can’t describe electron-electron interactions if there’s more than 2. can’t understadn the movement
Poincare’s theorem states “when multiple bodies interact, their equations of motion become coupled, and thus nonlinear. this means that the motion of one body depends on all the others in a complex way, making it impossible to find a general closed-form solution solution” what does this theorem mean for the use of the schrodinger equation?
can not get a close answer with the equation for a multielectron atom
based on poincare’s theorem, what species can schrodinger’s approach be used to obtain an exact solution
one electron species
since it is theoretically impossible for the schrodinger equation be used to obtain an exact solution for a species with two or more electrons, what must be done to solve the equation for any many-electron species
you must create many one electron problems
is it reasonable to expect that the calculated electronic structure of a species to be exactly the same as that found experimentally
no, its incredibly impossible to estimate exactly but can get close
if approximation to the schrodinger equation are made, what two conditions are required of the model
the model must have a unique solution and it must be practical
if approximation to the schrodinger equation are made, what to conditions are desirable for the model
it should preferably be variational and size consistent