Topic 2: Covalent bonding
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Define covalent bonding!
The sharing of outer electrons in order for atoms to obtain a full shell. There is an electrostatic attraction between shared electrons and positive nucleus.
What is dative covalent bonding?
When one atom donates 2 of its own electrons to another atom or ion to form a covalent bond
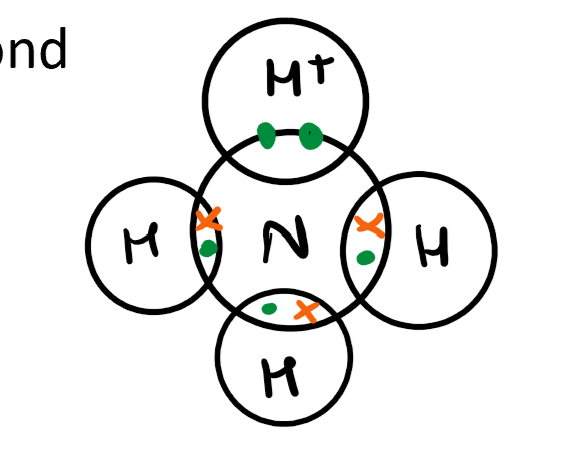
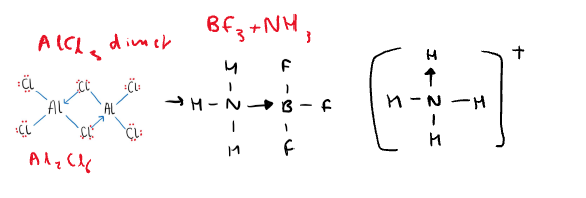
What are examples of dative covalent molecules? (Draw them out)
How does bond length affect bond enthalpy?
The shorter the bond the higher the bond enthalpy
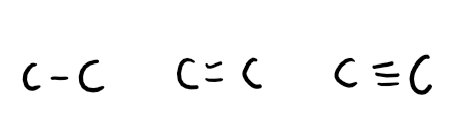
Out of these carbon to carbon bonds, which one has the highest bond enthalpy and why?
Triple bond C has highest bond enthalpy (attractive force refers to the electrostatic attraction between shared electrons and the positive nucleus)
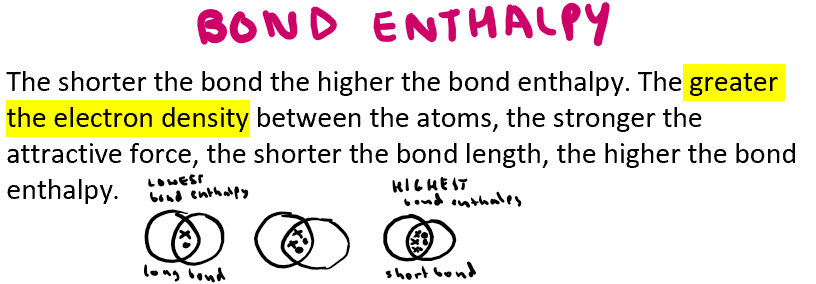
Why do 2 C-C have a stronger bond enthalpy than C=C?
2 C-C bonds are stronger as they contain 2 sigma bonds rather than C=C bond which contains a sigma and pi bond
What is stronger, sigma or pi bond?
Sigma bond
What bonds are present in simple molecules?
London forces
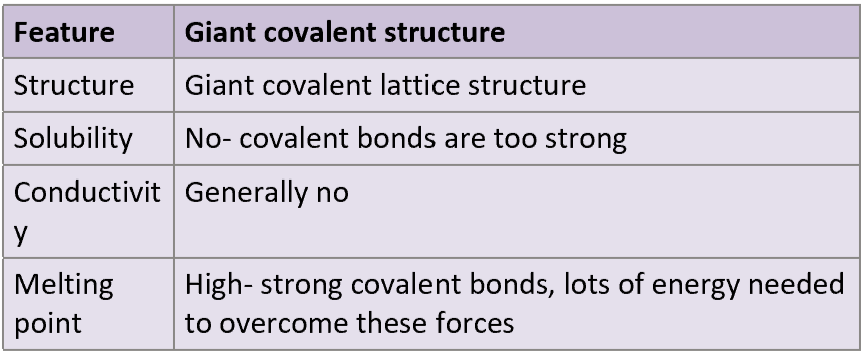
In general for giant covalent structures, what is its structure, solubility, conductivity and melting point? When possible explain the reasoning behind certain properties
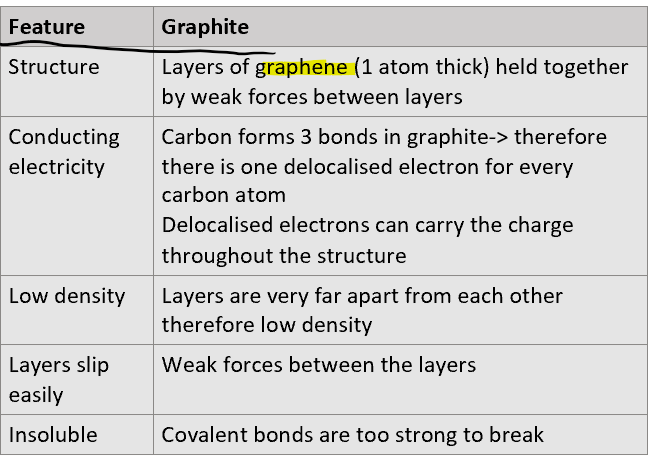
Describe: structure, conductivity, solubility and other properties of graphite and explain why when possible
Describe the properties of graphene

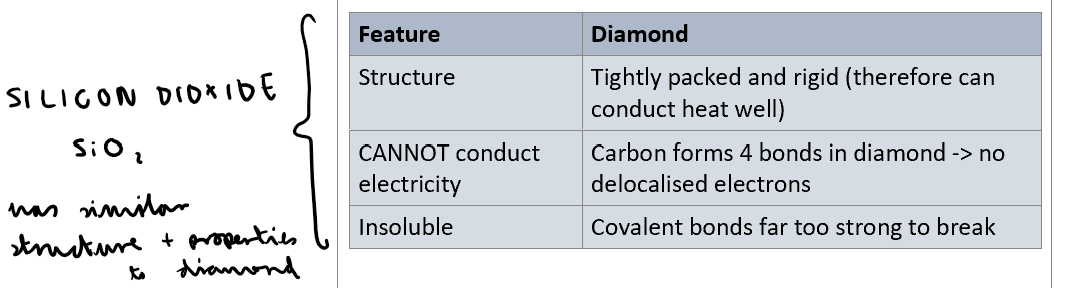
Describe structure, conductivity and solubility of diamond and silicon dioxide?