Lab 3 (Carbon NMR)
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
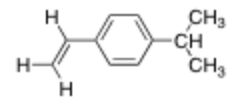
How many unique C NMR signals does this compound produce?
8 Signals
Terminal alkene carbon
Alkene carbon
Aryl carbon attached to alkene substituent
Equivalent carbons next to that
Aryl carbon attached to isopropyl group
Equivalent carbons next to isopropyl group
Methine carbon on isopropyl group
2 methyl carbons from isopropyl group
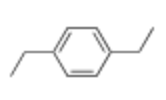
How many C13 NMR signals would this compound produce, give approximate chemical shift
4 Unique Signals
2 Methyl Carbons (8-35)
2 Methylene Carbons (15-5)
Aryl carbon attached to substituents (110-170)
2 equivalent aryl carbons next to substituent (110-170)

How would you distinguish between these 2 compounds using proton NMR?
The first structure would have a larger coupling constant than the second one since the protons are farther apart
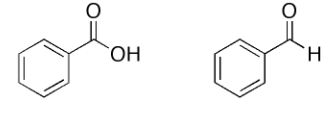
How would you distinguish these compounds based on C13 NMR. Give the most characteristic difference in their spectra
Main difference is the carboxylic acid and aldehyde group
Carboxylic acid has a chemical shift of 175-185
Aldehyde has a chemical shift of 190-200
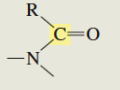
What is the C13 chemical shift for an amide?
165- 175ppm
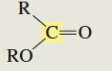
What is the C13 chemical shift for an ester?
165-175 ppm
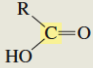
What is the C13 chemical shift for a carboxylic acid?
175-185 ppm
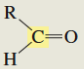
What is the C13 chemical shift for an aldehyde?
190-200 ppm
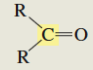
What is the C13 chemical shift for a ketone?
205-220 ppm (weak signal bc there is no protons)
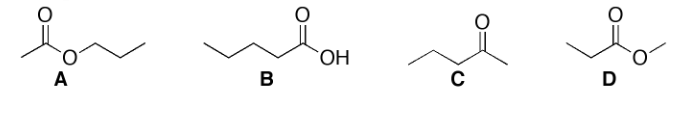
A C13 NMR Spectra has the following signals: 10.3, 20.7, 21.9, 65.9, 170.2 ppm. Which compound does this spectrum belong to? Justify your choice showing that the spectrum is consistent with the structure.
Structure A
5 unique signals
170 ppm signal from ester
65.9 ppm signal from C-O carbon
20.7/ 21.9 signals from either methylene or methyl carbon next to carbonyl
10.3 signal from methyl carbon farthest from any oxygens
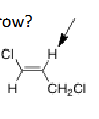
What should be the multiplicity of the proton at the arrow?
Doublet of triplets, this is because there are 2 different sets of nonequivalent neighboring protons
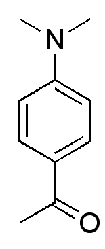
How many signals should be present in the 13C NMR of the following compounds?
7 unique signals
1 signal for 2 methyl carbons
1 Signal for aryl carbon attached to Nitrogen
Equivalent carbons next to that carbon
1 for aryl carbon attached to carbonyl group
Equivalent aryl carbons next to that carbon
Carbonyl carbon
Methyl carbon next to carbonyl
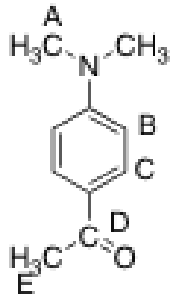
Which of the carbons in the following compound should appear at the value of highest chemical shift?
D, typically carbonyl carbons have high chemical shifts (ketones are the highest)
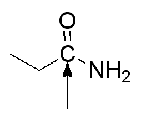
What should be an approximate chemical shift of the carbon pointed by arrow ?
~170 ppm (remember amide carbonyl is 165-175 ppm)