C200_ General Chemistry equation Sheet
1/118
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
119 Terms
Dilutions
M1V1=M2V2 or C1V1=C2V2
colligative properties
properties that depend on the concentration of solute particles but not on their identity
* Examples of these include boiling point depression, freezing point depression, osmotic pressure and vapor pressure
Amphoteric
Compounds that can react as both an acid and a base, depending on the reaction conditions
Percent error
(A-T)/T x 100
absorbance and beer lambert law
ecl (e=molar extinction coefficient; c=samples concentration; l= path length
*if you know the absorbance of a solution, you can determine its concentration
1 mol = how many atoms
6.023x10^23
combustion reaction
(hydrocarbon or alcohol) + 02 --> co2 to H20
Emprical Formula
most reduced form of a molecular formula
Energy of a photon equation
Planck's constant x speed of light / frequency (HC/F)
*H=6.63x10^-34
*c=3.0x10^8
f= photons frequency
quantum numbers
n (principle number), l(azimuthal), ml(magnetic), ms (spin)
*n= shell (distance from the nucleus)
*l= subshell (type of orbital)
-l=0 (s); 1(p); 2(d); 3(f)
*Ml=(-1....1) (determined by the l value )
ms= 1/2 & -1/2
paramagnetic vs diamagnetic
unpaired electrons attracted to magnets
dia: all paired and slightly repelled by magnets
relationship between energy, frequency and wave length
high energy = high frequency = low wave length
ionic bond properties
(metal + nonmetal)
generally have high melting & boiling points, tend to be hard, brittle solids; tend to conduct electricity only when melted or dissolved in water----not in solid state
covalent bond properties
(between two nonmetals)
sharing of electrons between with elements with similar electronegativity.
* high melting and boiling poiints, hard and do no conduct electricity
molecular bonds
(2 or more non metals) low melting points and do not conduct electricity
Ion size
more electrons = bigger size (anion) -
less electrons = smaller size (cation +)
isoelectronic series
comparing elements with the same number of electrons.
The compound with the smallest amount of protons will be the largest in size
ionization energy
The amount of energy required to remove an electron from an atom
trend: like electronegativity but includes noble gasses
electronegativity
how thirst an atom is for electrons
up and to the right excluding noble gasses
electron affinity
the energy given off when an atoms gains an elecctron (exothermic reaction)
*Cl has highest electro negativity
temperature c to k
k= c+ 273
Volume
1cm^3=1ml =1cc
pressure
force/area (1atm = 760 torr or mmhg)
Ideal gases behave
most ideally under low pressures and high temperatures
at STP 1 mole of any gas =
22.4 L
boyles law (gas law)
V is inversely related to pressure. ( V= 1/p)
Charles law (gas law)
V and temperature are related
Avagadro's Law
V=N
Combined Gas Law
P1V1/n1T1 = P2V2/n2T2
Ideal gas law
PV=nRT
R=.0821
At STP
P=1atm and T =273k
Density
P(MM)/RT = m/v
r=.0821 L*ATM/ mol k
Dalton's Law of Partial Pressures
Total pressure of a gas is equal to the sum of the partial pressure of the component gases
Ptotal= Pa + Pb...
In terms of gas A : Pa=Xa P total
Xa= mol fraction of gas A
Graham's law of effusion
Effusion refers to the movement of gas particles through a small hole. Graham's Law states that the effusion rate of a gas is inversely proportional to the square root of the mass of its particles.
r1/r2 = √(M2/M1) M=mola
r mass
r=rate of effusion
intramolecular forces vs Intermolecular forces
Intramolecular forces are the forces that hold atoms together within a molecule.
Intermolecular forces are forces that exist between molecules.
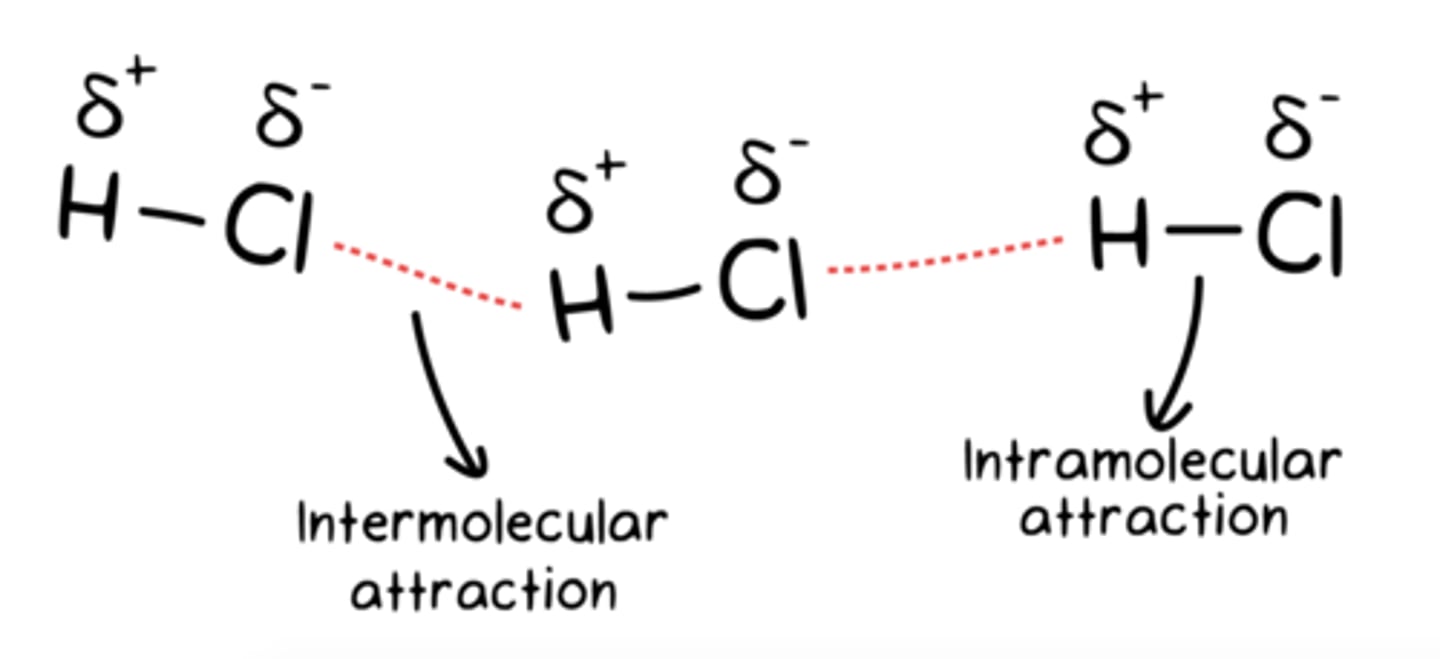
intermolecular forces
are forces that exist between molecules.
1). hydrogen bonding (strongest)
2) dipole dipole
3) dispersion forces (london disp/ van der wall) (weakest)
hydrogen bonding
Hydrogen atom bonded to either an O, N, or F
Dipole-Diople Forces
NON METAL molecules with polar bond causing the molecule to have a partial negative and positive charge
They line up in a complementary to each other and have big electronegativity differences between the two
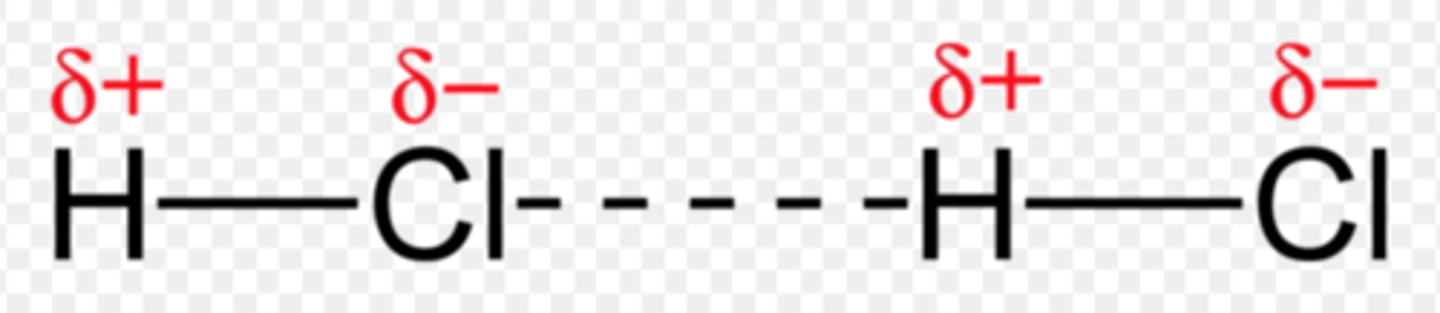
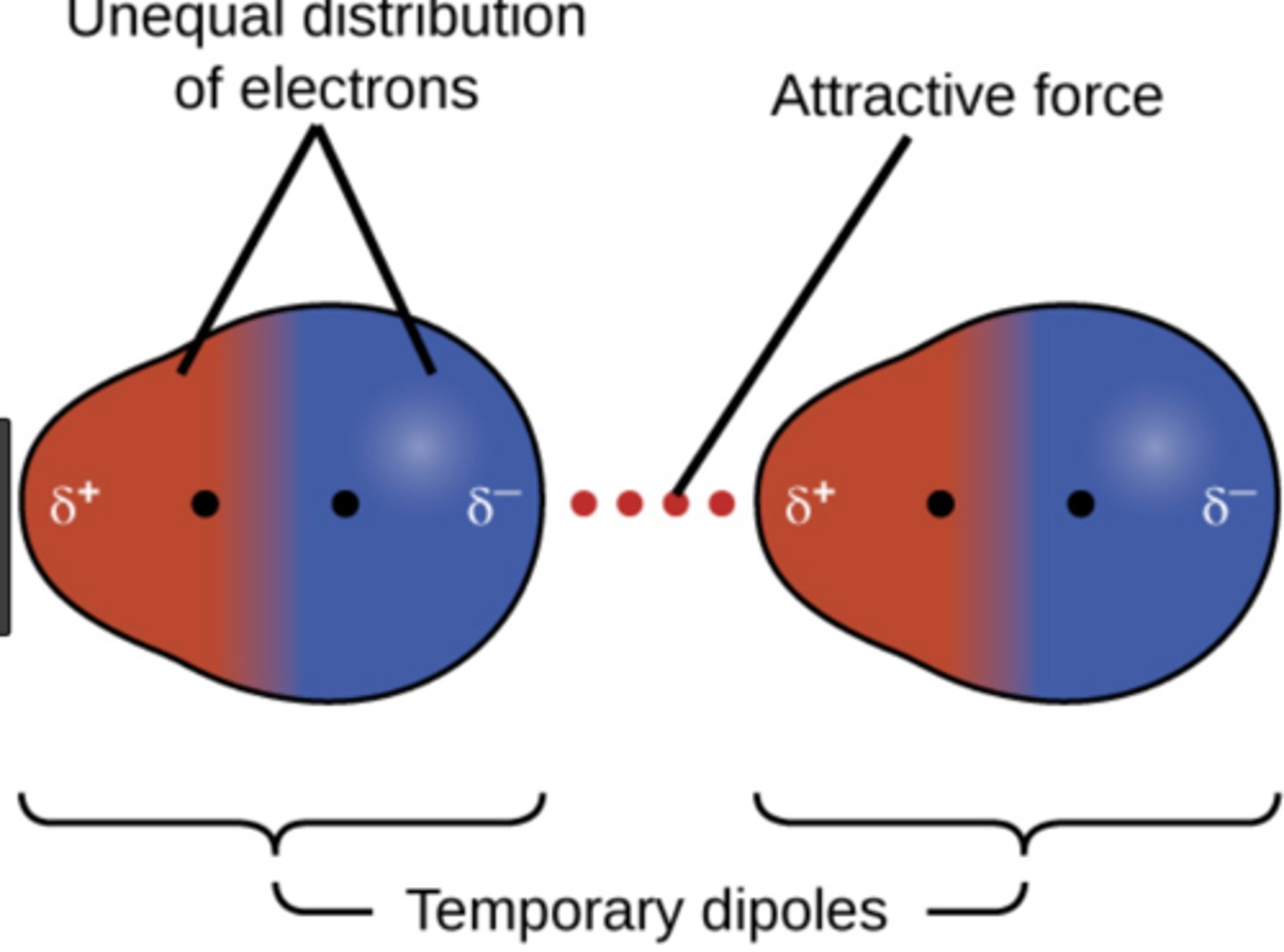
dispersion force
no significant difference in electronegativity
Dispersion forces are in all molecules and are responsible for molecules boiling points
intermolecular forces and boiling points
responsible for boiling points. stronger the intermolecular forces leads to :
1) higher boiling point
2)high heat of vaporization
3) higher viscosity
4) higher surface tension
5) LOWER vapor pressure
Intramolecular forces
forces are the forces that hold atoms together within a molecule.
Metallic bonds, Ionic bonds, Polar covalent bonds, covalent bonds,
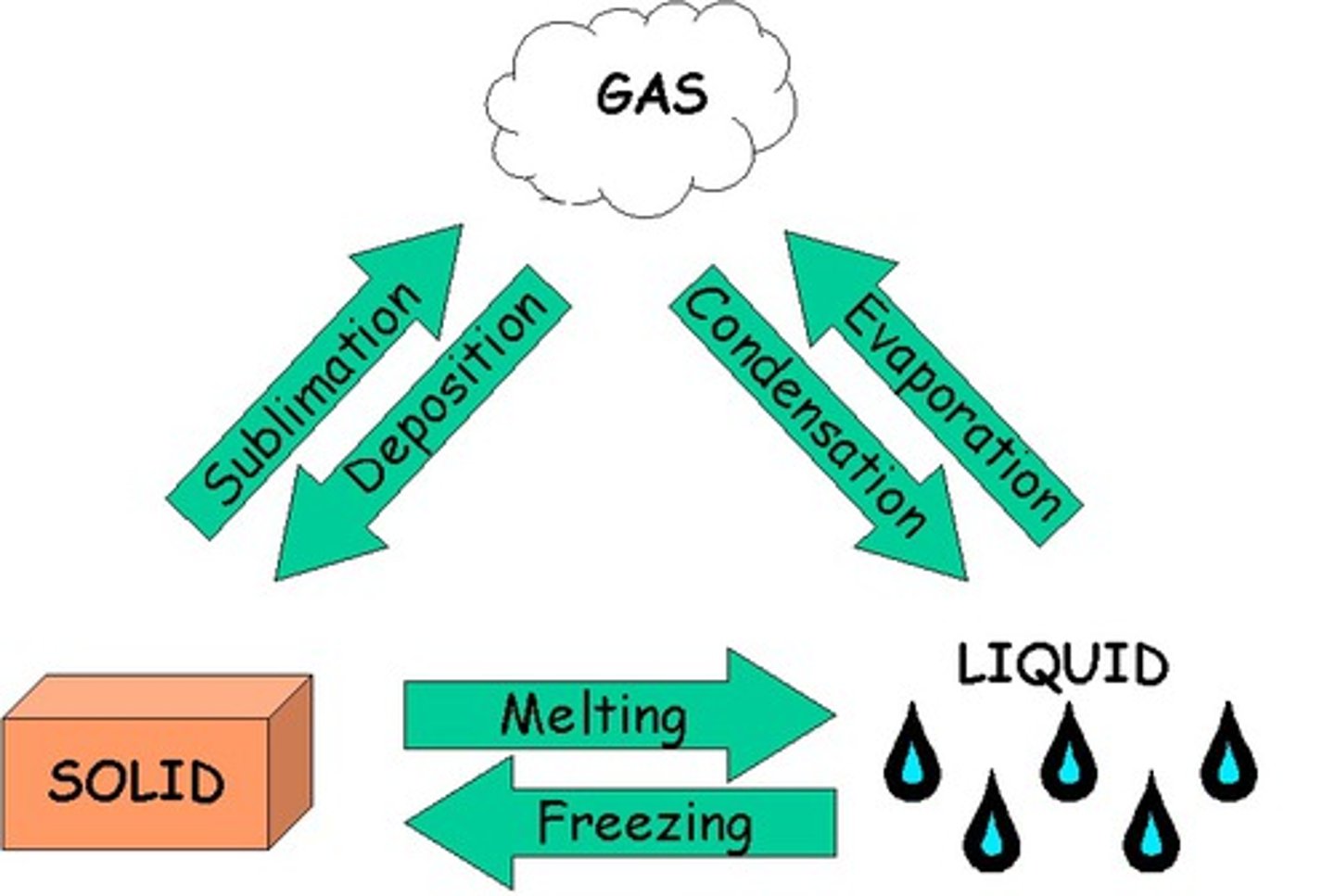
Phase changes
Freezing: crystalization
melting : fusion
Sublimation; fusion and vaporization : consumer heat therefore are endothermic reactions with a positive +ΔH and +ΔS (entropy, increasing disorder)
Deposition; condensation; crystallization are exothermic -ΔH because they release heat and have a -ΔS (because more order is being established)
Boiling point and vapor pressure
boiling point is when the vapor pressure = external pressure.
high boiling point = low vapor pressure
Phase Diagram - triple point
unique for each substance, point where all three phases are in equilibrium
Phase diagram - critical point
Point where liquid and gas are no longer distinguishable
Molarity (M)
moles of solute/liters of solution
Molality (m)
moles of solute/kg of solvent
Solubility of ionic compounds in water rules
most group one metal cations NO3-; clo4-;c2h302- and nh4+ salts are soluble
most ag2+; pb2+; s2-: oh-; hg2 (2+); co3 2-; po4 2- are insoluble
Solubility of solids and gasses (henrys law)
Pa=Kh(A)
pa= partial pressure of gas a
Kh= henerys law constant; changes with each problem
A= concentration of gas a
Freezing point depression
ΔTf= -iKfm
i= vant hoff factor
Kf= freexing point depression constant
m= molality
Boiling point elevation
ΔTb=- iKbm
i= vant hoff factor
Kb= boiling point depression constant
m= molality
Vapor pressure depression
Psoln=X solv P solv
Psoln= new VP of solution
x solv = mol fraction of solvent (percent of solvent in new solution)
P solv = VP of pure solvent
Osmotic pressure
pi=iMRT
i= vant hoff
M= molarity
r= .0821
T= temp
Pi= (n/v)RT
(n/v) = M
chemical kinetics
the study of how fast a reaction occurs or the reaction rate
Thermodynamics
tells us whether a reaction will occur but not how fast or slow it will occur
General Rate rate
rate= k (A)^m (B)^n
k= rate constant
m&n: determined experimentally
Rate constant units
Zeroth order: M/s (m^1 x s^-1)
First order: 1/s ( x s^-1)
Second order: 1/ M *S (m^-1 x s^-1)
Third order: 1/ M^2 *s (m^-2 x s^-1)
Integrated rate law units (Graphs)
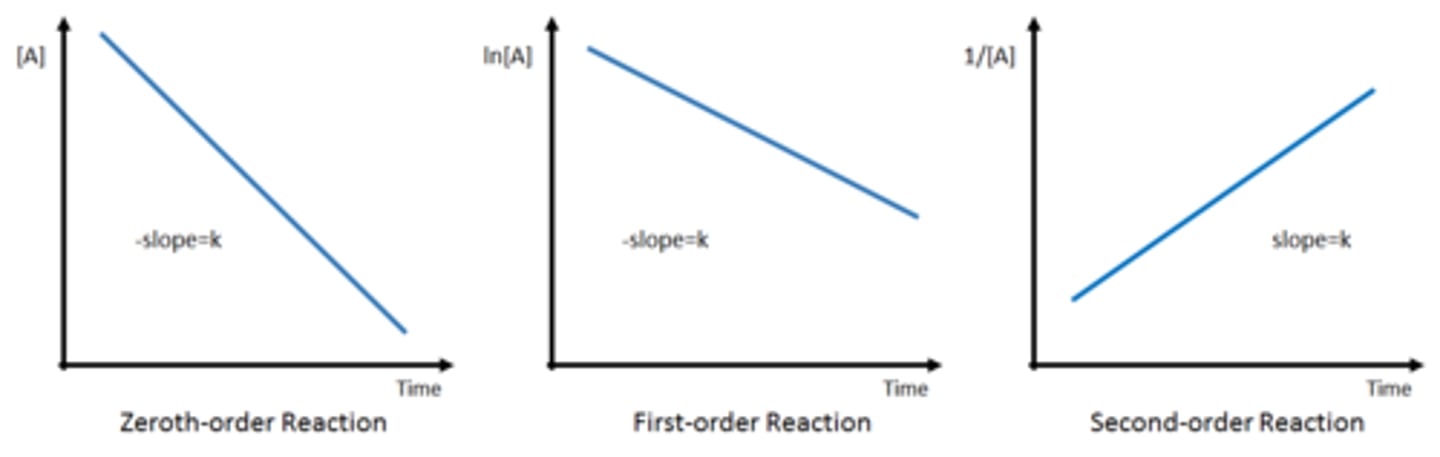
Collison theory and arrhenius equation
K = Ae ^-Ea/RT
ea= activation energy
A= frequency factor
r= 8.314 J/mol k
k=rate constant
Equilibrium constant expressions
Kc & Kp = Products/ reactants
Keq= kForward/ kreverse
Keq Rules
K>1 = products are favored at equilibrium
k<1= reactant are favored at equilibrium
solubility product constant (Ksp)
Ksp= products / reactants
pH & pOH
pH = -log (H+)
pOH= -LOG (oh-)
ph +poh = 14
(h+) & (oh-)
(H+)= 10^-PH
(OH-)= 10^-POH
(H+)(OH-)= 1*10^-14
Weak acids
HA + H2O <----> h30+ + A-
ka= (h30+)(A-)/ (HA)
*ka= acid dissociation constant
(H+) = SR(ka)(HA)
Weak base
A- + H2O <----> HA + OH-
kB= (HA)(OH-)/ (A-)
*kB= BASE dissociation constant
(OH-) = SR(kB)(A-)
pKa and pKb
pKa=-log(ka)
pKb=-log(kb)
pka+pkb = 14
Kw=ka x kb =1
Ka and Kb strength
larger Ka = smaller pka = stronger acid
larger kb= smaller pka = stronger base
Arrhenius acid
h+ donor in water
arrhenius base
OH- donor in water
Bronsted-Lowry acid
h+ donor
Bronsted-Lowry base
H+ acceptor
lewis acid
electron pair acceptor
lewis base
electron pair donor
conjugate acid-base pair
acid --> base (remove hydrogen and the charge decreases by one_
base---> acid (adds 1 hydrogen and increases the charge by 1)
Trends in acid strength
the more oxygen atoms means the more acidic it is due to resonance.
if the number of oxygens is the same the the more electronegative heteroatom = more acidic
Neutralization reactions and normality
*reactions that occur between acids and base; which always makes h20 and a salt
NaMaVa=NbMbVb
Na= number of hydrogen groups the acid can donate
Nb= number of oh groups the base can donate
Buffers and henderson hasselbalch
buffer= is a solution that resists ph change. it is made from a weak conjugate acid/ conjugate base pair
ph= pka +log (A-)/(HA)
Enthalpy
the amount of heat energy a substance contains
HA + H2O <----> h30+ + A-
ΔH > 0 -endothermic
ΔH < 0 exothermic
Enthalpy of formation (ΔHf)
Δhf = nΔH(products) - nΔH(reactants)
n= coefficient from the balanced reaction
first law of thermodynamics (law of conservation of energy)
ΔE= q + w
q= heat
w=work
ΔE= change in internal energy
pressure-volume work
w= -p Δ V
ΔV= change in volume
p= external pressure
calorimetry thermal energy
q= -Ccalorimetry ΔT
Cal= specific heat of calorimeter
heat curves and thermal energy
q=mc ΔT or q=mc ΔH(fusion or vaporization)
m=mass
c= specific heat
ΔT= change in heat (final - initial )
entropy formula
a measure of how disordered something is
ΔS= Sum(n)Sproducts- Sum(n) S reactants
Sgas> Sliquid> Ssolid
saq> Ssolid
Srxn> 0: when there is an increase in the number of moles of gas
Bond Disociation Energy
ΔH= Sum ΔH (products) - S ΔH (reactants)
=Sum ΔHbroken - Sum ΔH formed
making bonds = exothermic (ΔH-)
breaking bonds= endothermic (ΔH+)
Gibbs free energy equation
a measure of spontaneity
ΔG^0 = ΔH - TΔS
ΔG^0= standard conditions
ΔH= enthalpy
ΔS= entropy
T= temperature in kelvins
Gibs free energy equation (Keq standard and nonstandard conditons)
ΔG= ΔG^0 + R(T)(Ln Q)
ΔG^0= -RTLnKeq
ΔG^0= standard conditions
Keq= equilibrium constant
Q= reaction quotient
R= 8.314 J/mol k
standard cell potential (E°cell) (reduction potentials)
E^o cell = E^o reduction + E^o oxidation
E^o = E^o cathode + E^o anode
Gibs free energy (ΔG) rules
ΔG= - (spontaeous)
ΔG= + (nonspontaenous)
ΔG= 0 at equilibrium
Gibs free energy relation with enthalapy (ΔH) and entropy (ΔS)
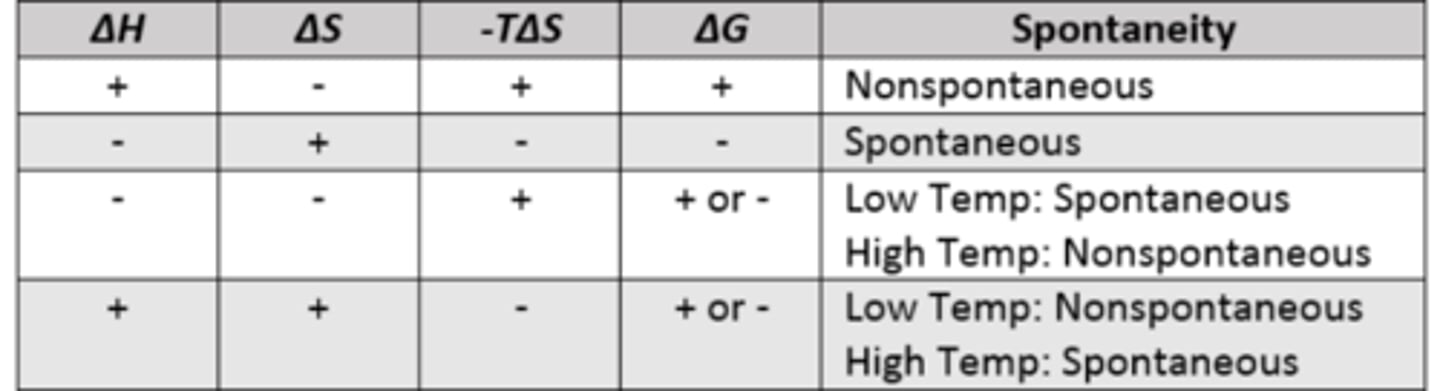
Relating Gibbs Free Energy and K

Redox reactions
Reactions that involve the transfer of electrons from one element to another.
Reduction-oxidation reaction
(OIL RIG)
-3------> 3 (OXIDATION)
3+-------> 3 (REDUCTION)
Reducing agent (reductant) in redox reactions
is the element that GIVE UP e- (gets oxidized) is the REDUCING AGENT
Oxidizing agent (oxidant) in redox reactions
is the reactant that gets reduced
Balancing redox reactions
Break the reactions into (1/2) reactions
1 should be the oxidation reaction and the other should be the reduction equation
anode
is the site of oxidation
cathode
is the site of reduction
Balancing Redox Reactions (under acidic conditions)
1). identify what is being oxidized and what is being reduced
2). seperate the oxidation reaction from the reduction reaction (1/2) reactions
3). balance all the atoms the ARE NOT HYDROGENS OR OXYGENS
4). balance the oxygens by adding H2o to which side needs it
5). balance the hydrogens by adding h+ to the side the needs it
6). add e- wherever necessary to balance the charge on each side of the equation
7). as needed, add integer to your half reactions to make the number of moles of e- equal in each of your half reactions.
8). the final balanced number of e-s is the number of overall electrons per mole that get transferred in this particular redox reaction
Balancing Redox reactions (under basic conditions)
1). identify what is being oxidized and what is being reduced
2). seperate the oxidation reaction from the reduction reaction (1/2) reactions
3). balance all the atoms the ARE NOT HYDROGENS OR OXYGENS
4). balance the oxygens by adding H2o to which side needs it
5). balance the hydrogens by adding h+ to the side the needs it
6). add the same number of OH- to both side of the equation that you added to your half reactions.
- combine the h+ and oh- on the same side of the equation to form H2o. and eliminate excess h20
7). add e- wherever necessary to balance the charge on each side of the equation
8). as needed, add integers to your half reactions to make the number of moles of e- equal in each of your half reaction.
9) the final balanced number of e-s is the number of overall electrons per mole that get transferred in this particular redox reaction