Quantum Numbers and Electron Configuration
1/9
Earn XP
Description and Tags
12-Pro Chemistry '24-'25 (made with AI assistance.)
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
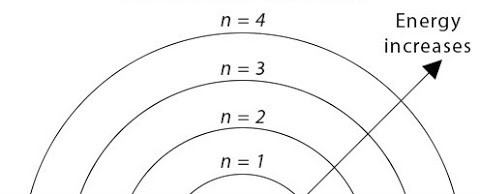
Principal Quantum Number
n; n ≥ 1 - where each number represents an energy level
Responsible for overall size of the orbital
Azimuthal Quantum Number
l
Responsible for overall shape of orbital
Also known as the angular momentum number
Ranges from 0 to n - 1
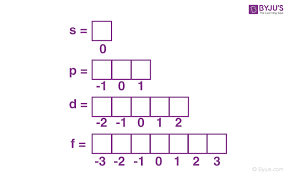
Magnetic Quantum Number
Responsible for orientation of the orbital
Ranges from -l to +l, including 0
Spin Quantum Number
Responsible for the spin of the electron
Takes on a value of +1/2 or -1/2
Types of Orbital Shapes
s; where l = 0
p; where l = 1
d; where l = 2
f; where l = 3
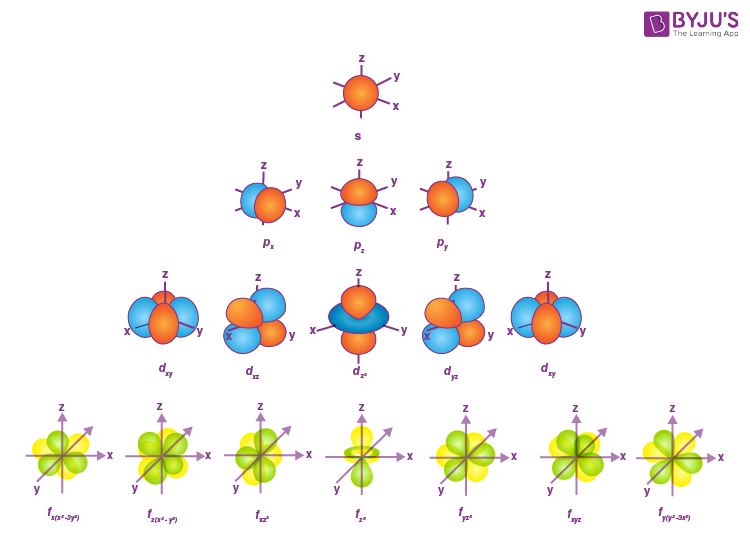
Some mnemonics for Orbital shapes
s - Spherical
p - Pretzel or Parang Dumbbell
d - Double Pretzel or Dumbbell
f - Fucked up (sir’s words)The sign for the letter S in ASL is a closed fist, so using this each finger you put up corresponds to an orbital shape as per n values
s=closed fist, p= 1 finger, d=2 fingers, f=3 fingers
Aufbau Principle
Electrons occupy the lower energy orbitals first, unless electrons are excited.
“Quantum Ladder”
Pauli Exclusion Principle
No two electrons can have the same quantum numbers.
Each orbital can hold a maximum of two electrons with opposite spins.
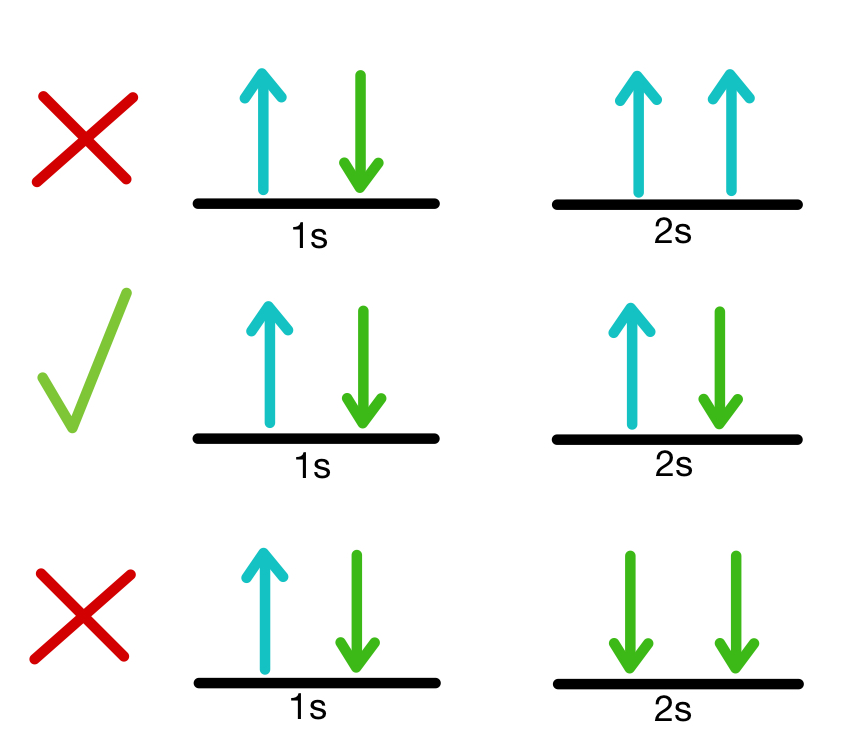
Hund’s Rule
Electrons fill orbitals singly before pairing up to minimize repulsion.
Orbital is stable when as many electrons have the same spin.

Mnemonic for Electron Configuration
Si Sharon Pumasok Sa Pinto Sa Door Pinto Sa Door Pinto Sa Front Door Pinto Sa Front Door Pinto
