Lab 4 Chemical Tests
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
Solubility Test
soluble in pure water - small molecules
soluble in 5% NaHCO3 (weak base) - carboxylic acids, small molecules
soluble in NaOH (strong base) - phenol, small molecules
soluble in HCl (acid) - amine, small molecules
insoluble - aldehyde, ketone, ester, alcohol
Cerium Test
goal - test for alcohols or phenols
looking for pronounced color change (specifically red)
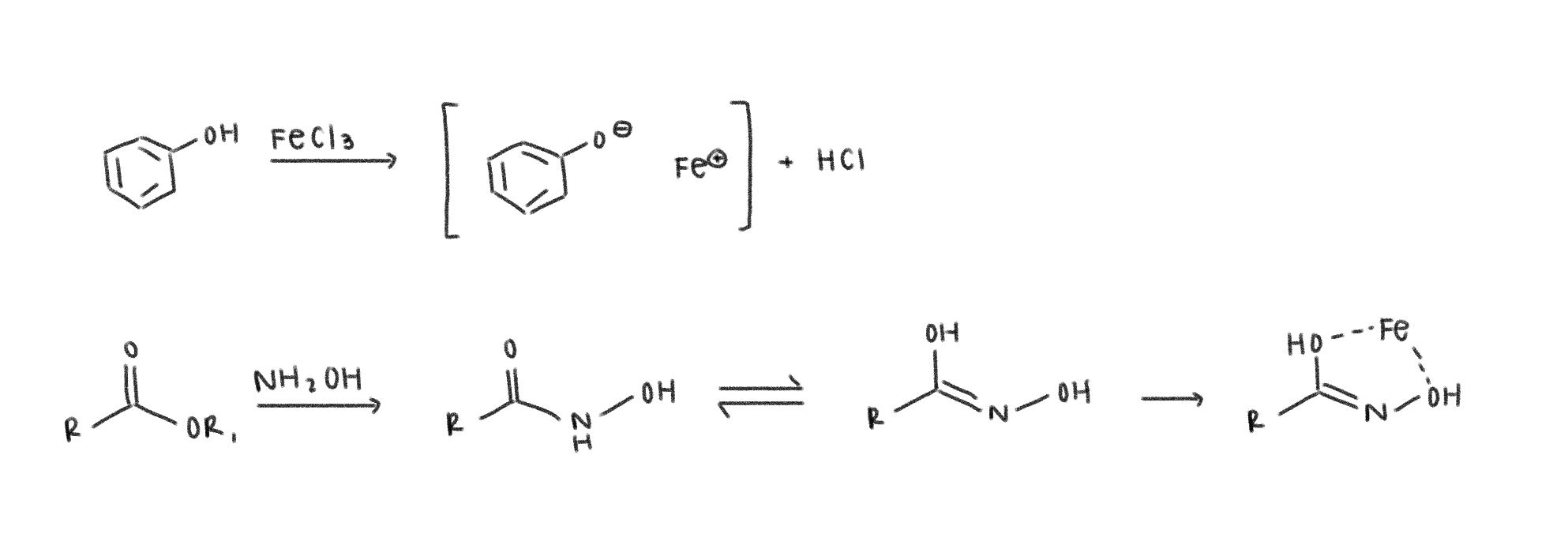
Ferric Test
goal - test for phenols or esters
part 1 - tests for phenols
part 2 - tests for esters
looking for pronounced color change in both for positive result
*carbonyls that easily tautomerize to enols can give false positive
Tollens Test
goal - test for aldehydes
oxidation of aldehydes to carboxylic acid, silver precipitates as solid “silver mirror”
looking for silver mirror on glass
aldehyde + Ag(NH3)2 ——> carboxylic acid + Ag(s)
Nitrous Acid Test
goal - test for amines & type of amine
primary alkyl amines - immediately release N2 gas, even at cold temp
primary aryl amines - release N2 gas when warmed
primary aryl amines can be converted to diazonium ion then azo dye (color change)
secondary amines form yellow nitroso compound
*ketones or phenols can sometimes give a false positive
Oxime Test
goal - test for aldehydes and ketones, only done if other tests were inconclusive
carbonyl reacts with NH2OH to form an oxime
*some easily oxidized alcohols (allylic/benzylic alcohols) can give a false positive since they oxidize to carbonyls (ketones, aldehydes, carboxylic acids)