Chem 40A Midterm 1
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
70 Terms
alkane
single bond
alkene
double bond
Alkyne
triple bond
Alcohol
R-OH
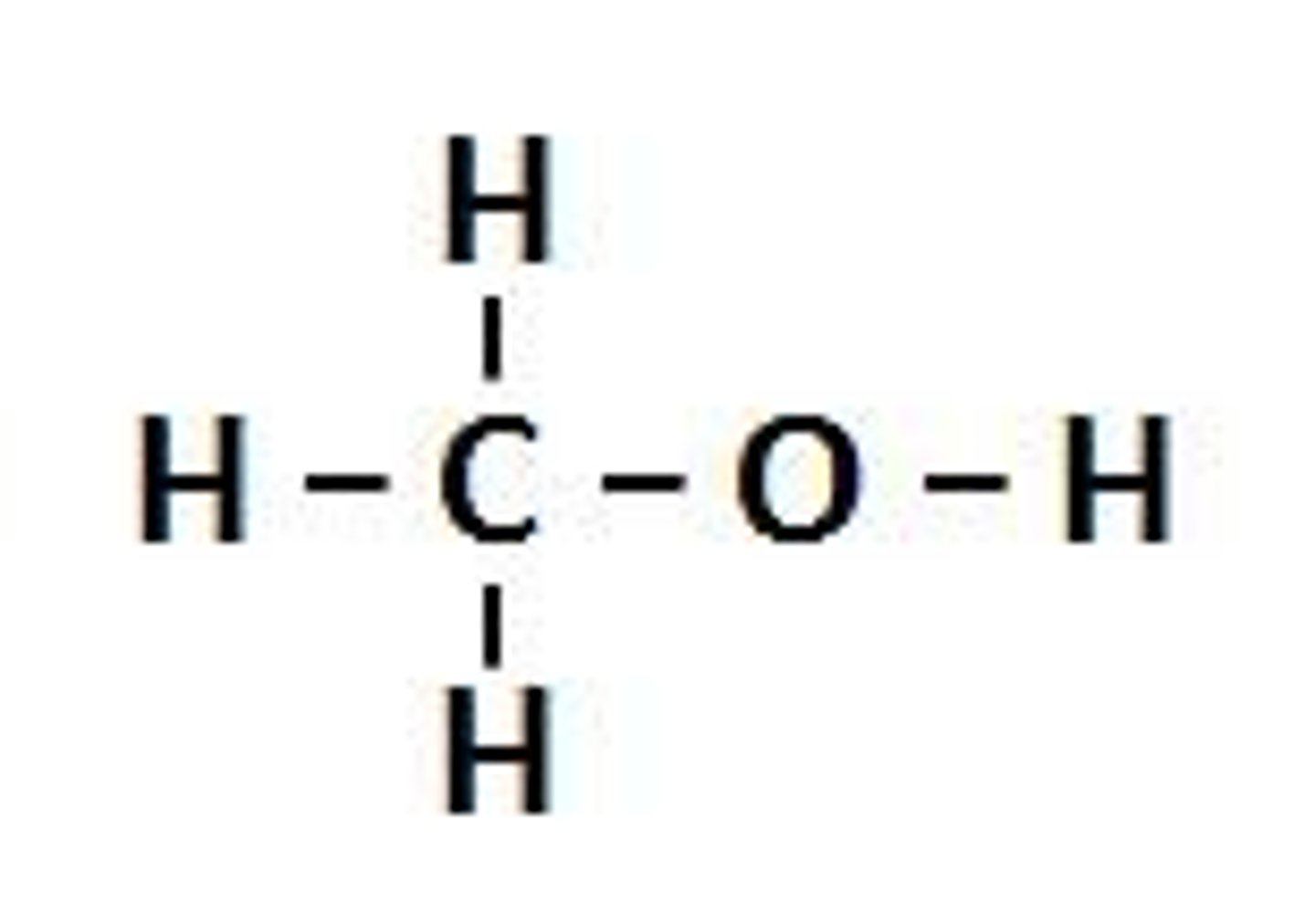
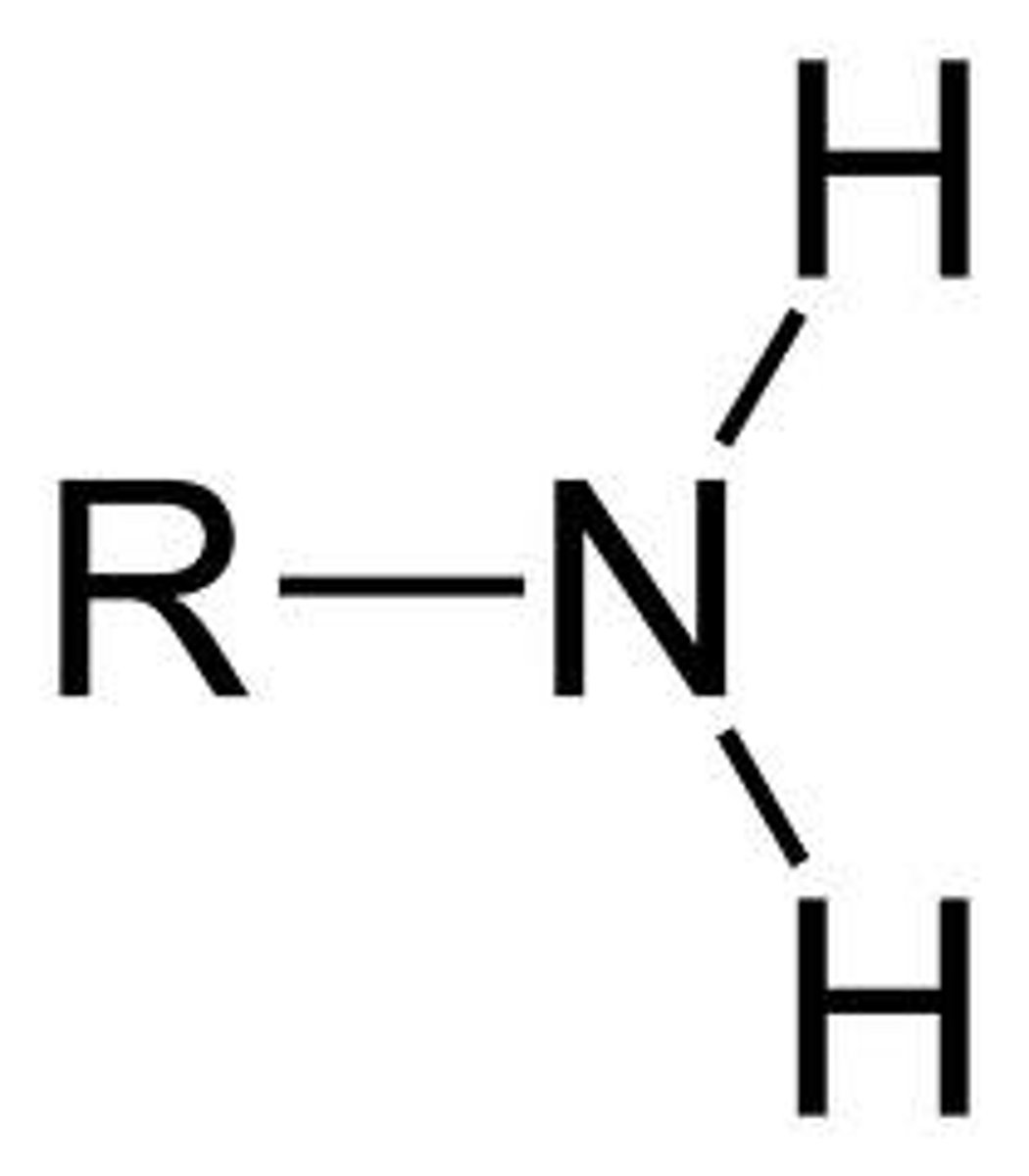
Amine
R-NH2
alkyl halides
organic compounds in which one or more halogen atoms- fluorine, chlorine, bromine, or iodine- are substituted for one or more hydrogen atoms in a hydrocarbon
ether
C-O-C

Ketone
2 carbons attached to the C of the C=O
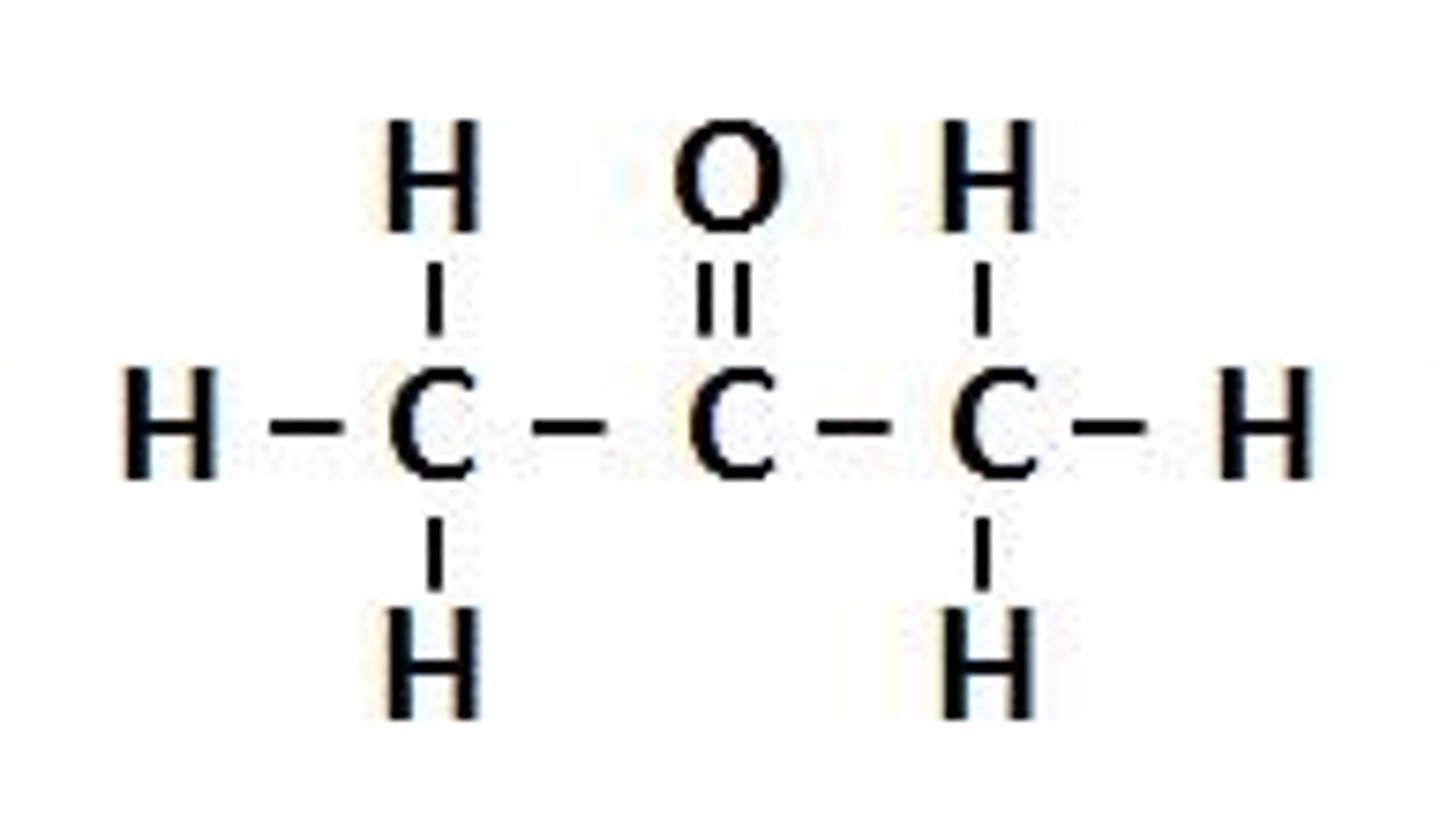
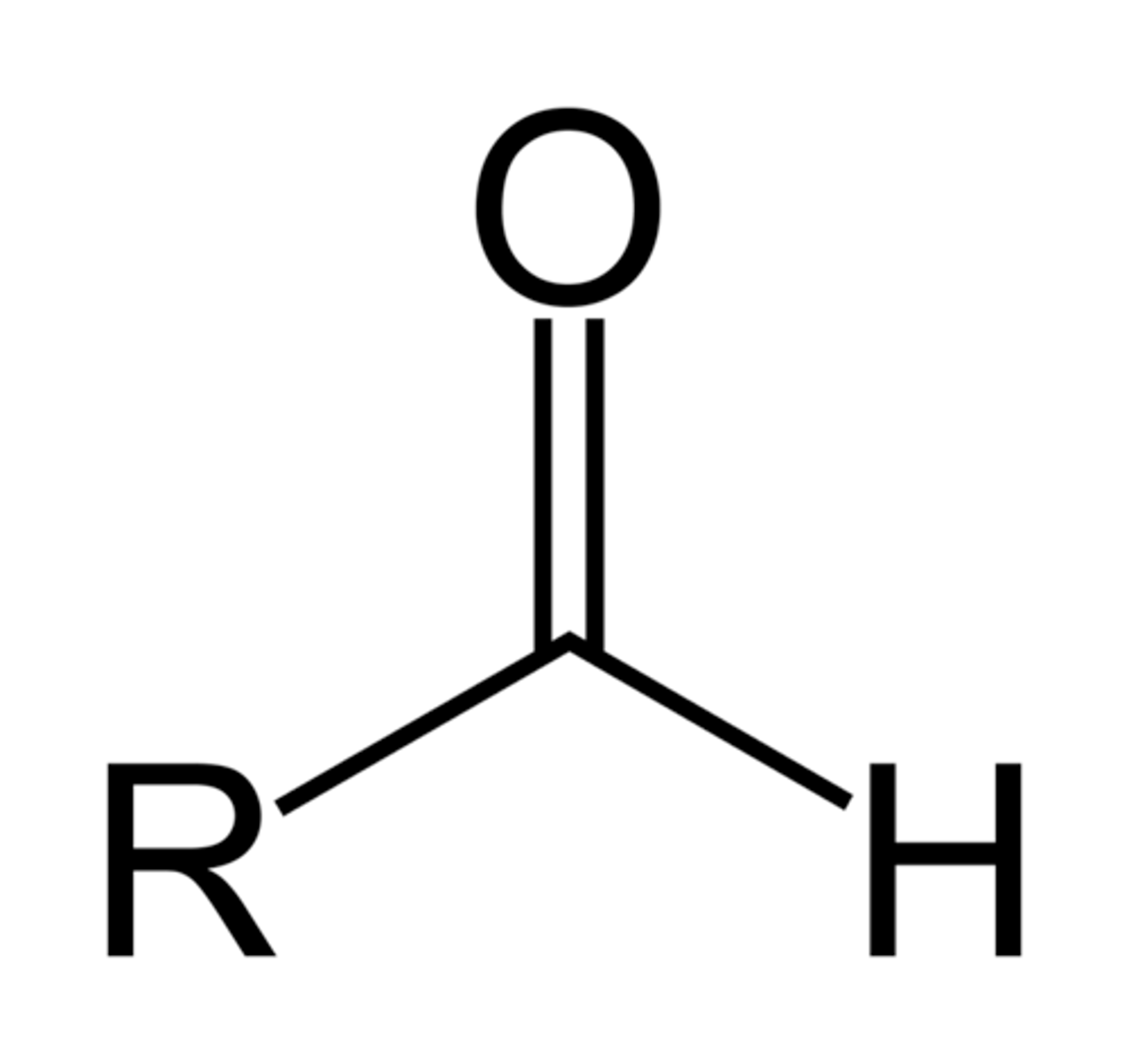
Aldehyde
at least 1 H attached to the C of the C=O
Aromatic groups/Arene
ring
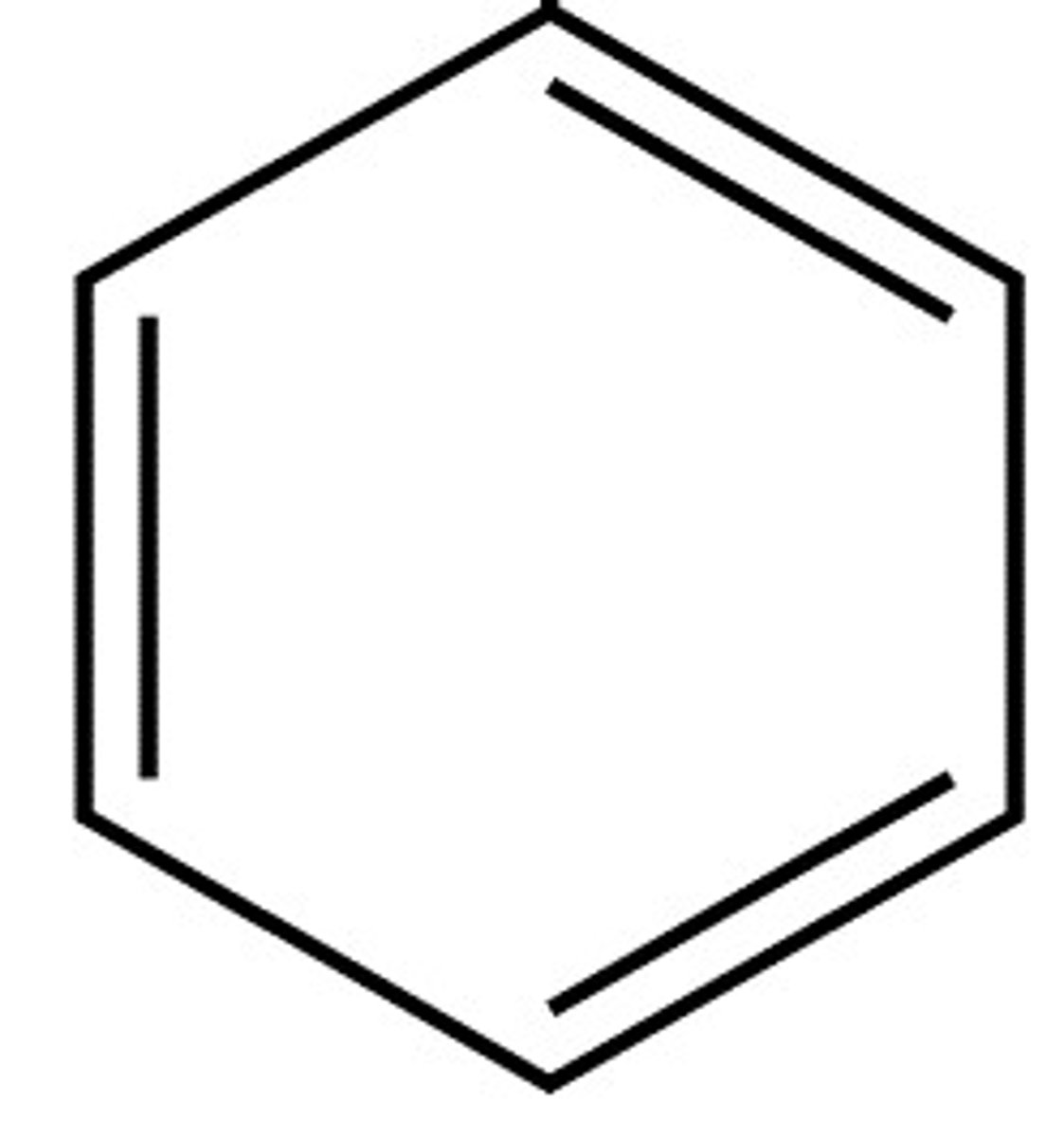
carboxylic acid
R-COOH
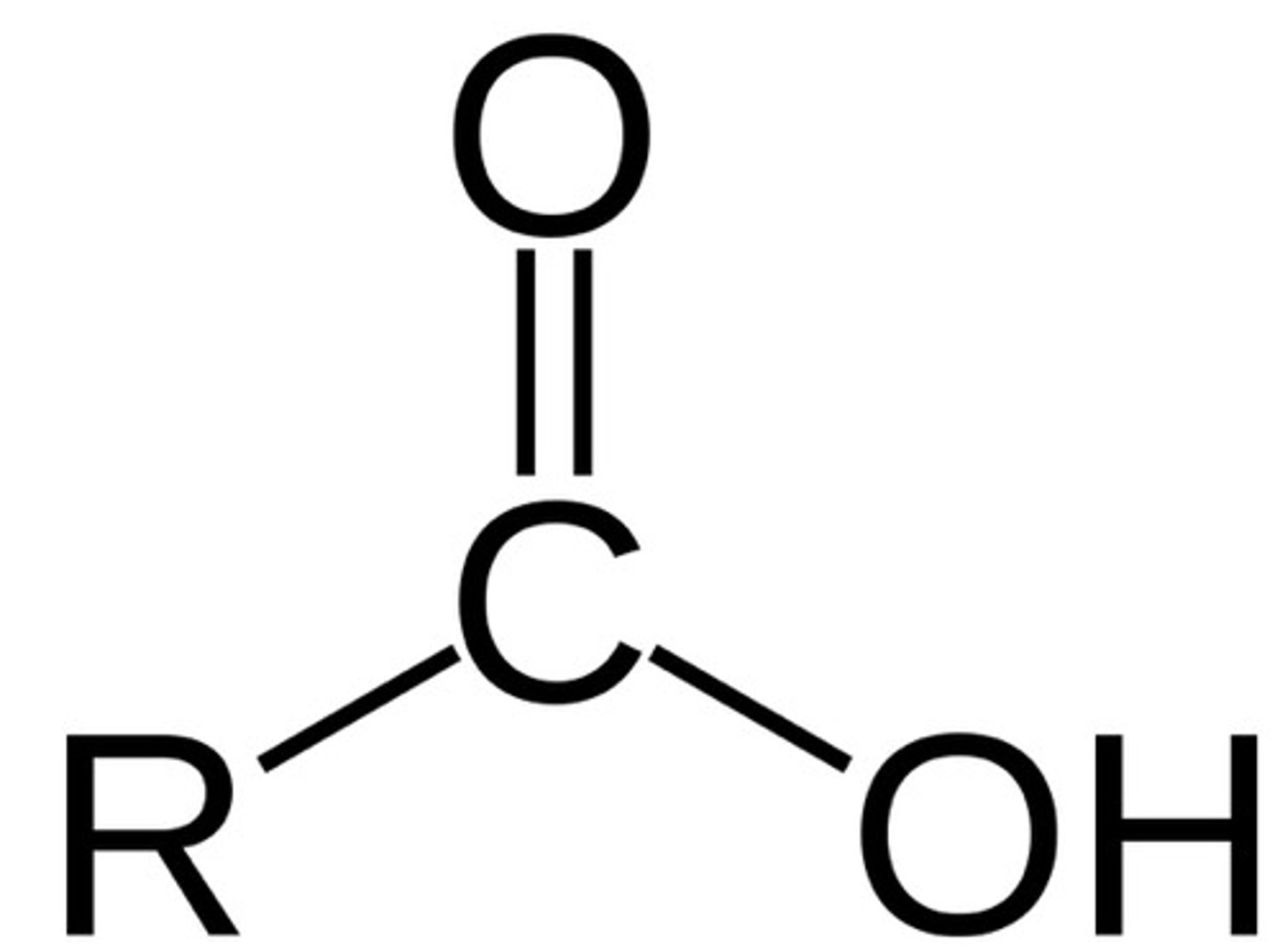
amide
R-CONH2
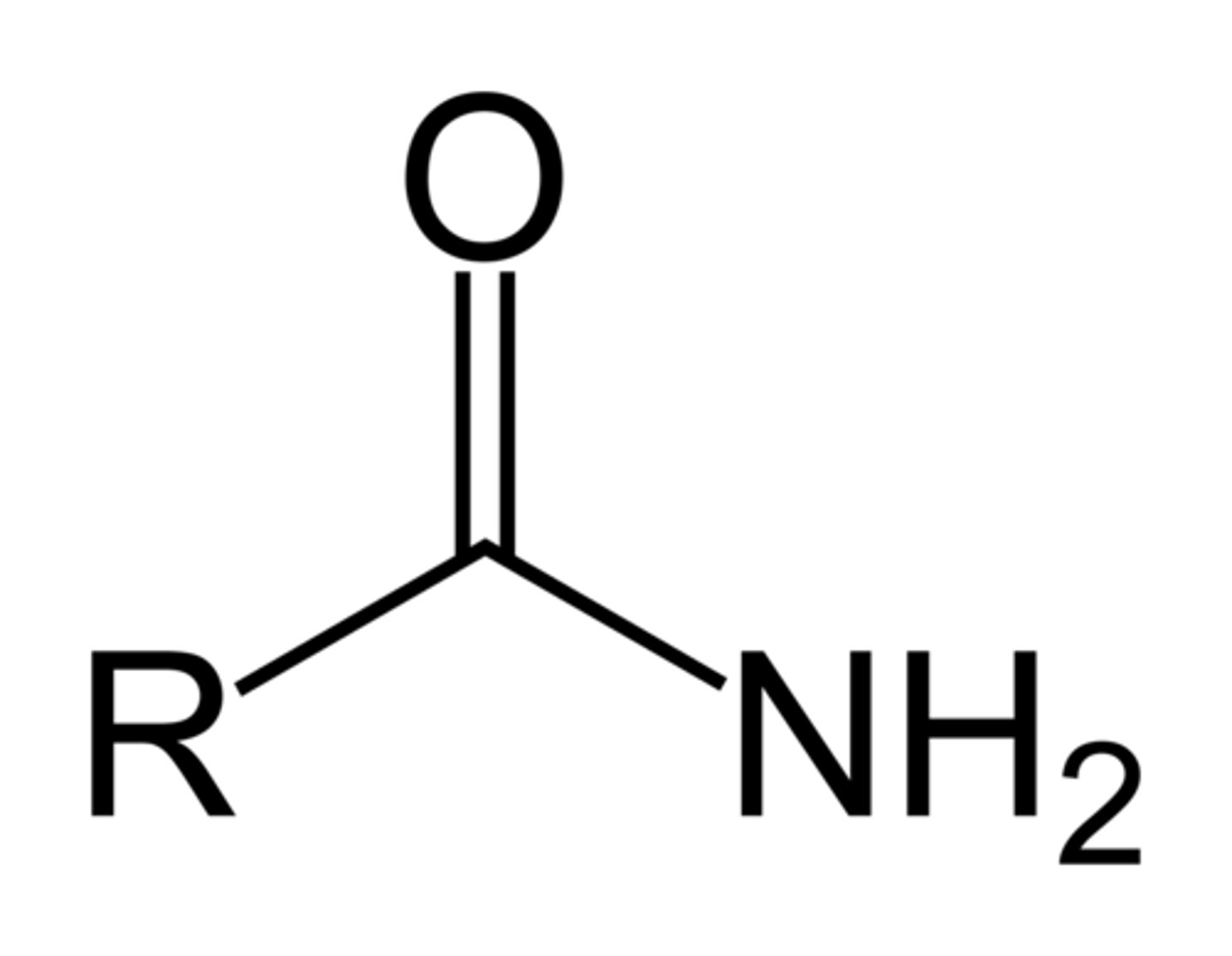
thiol
R-SH
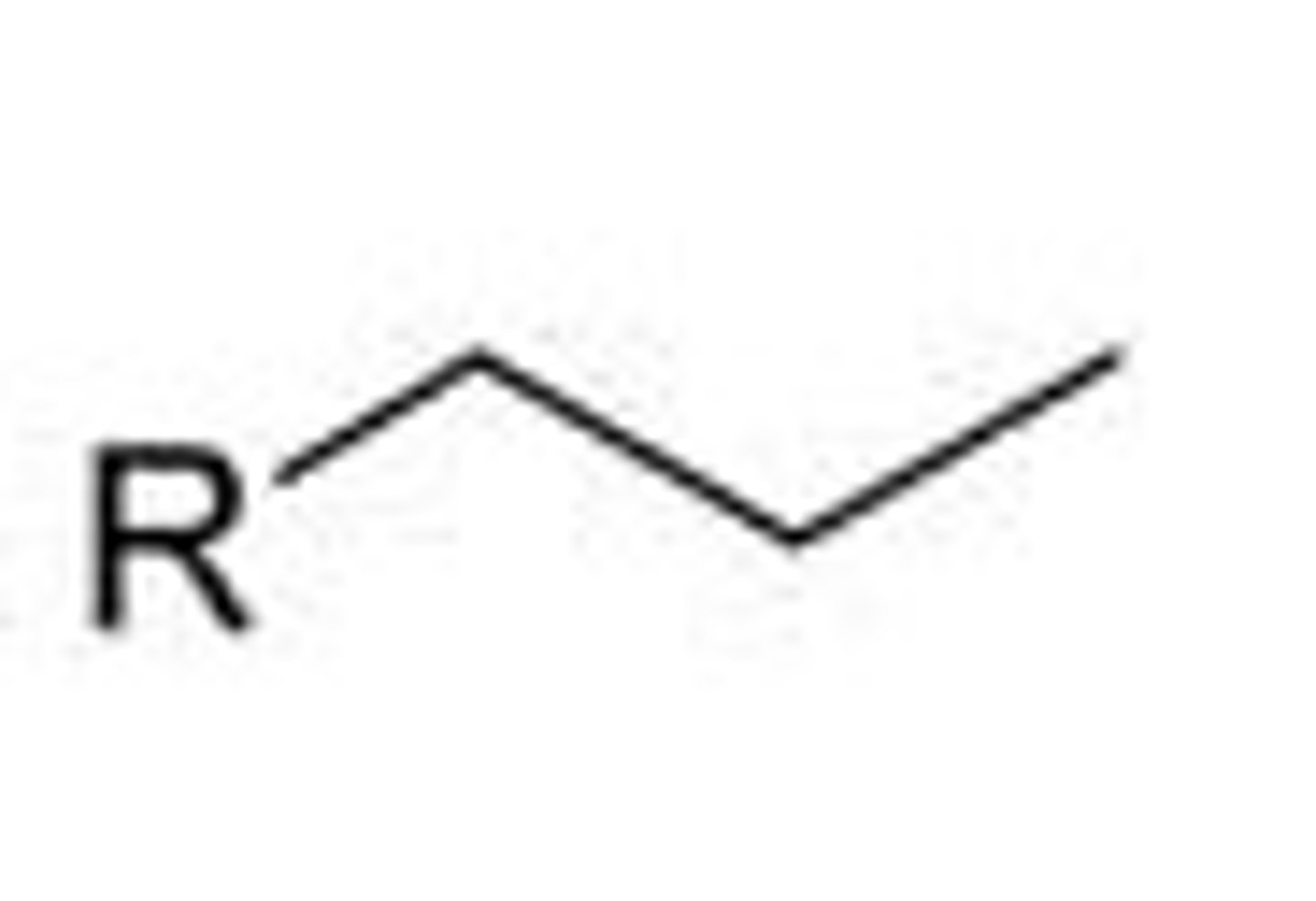
Propyl
3 Carbons
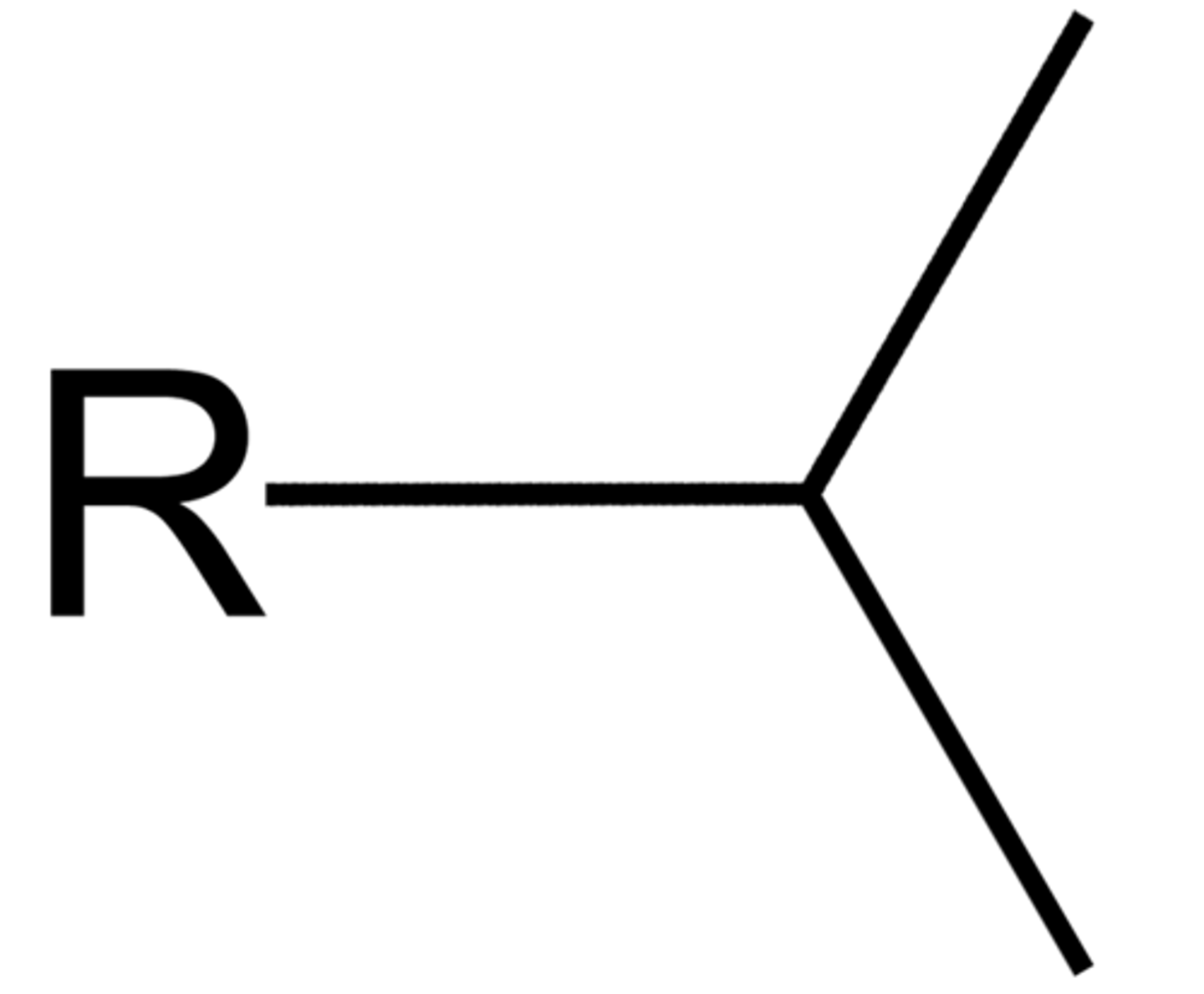
Isopropyl
1-methylethyl
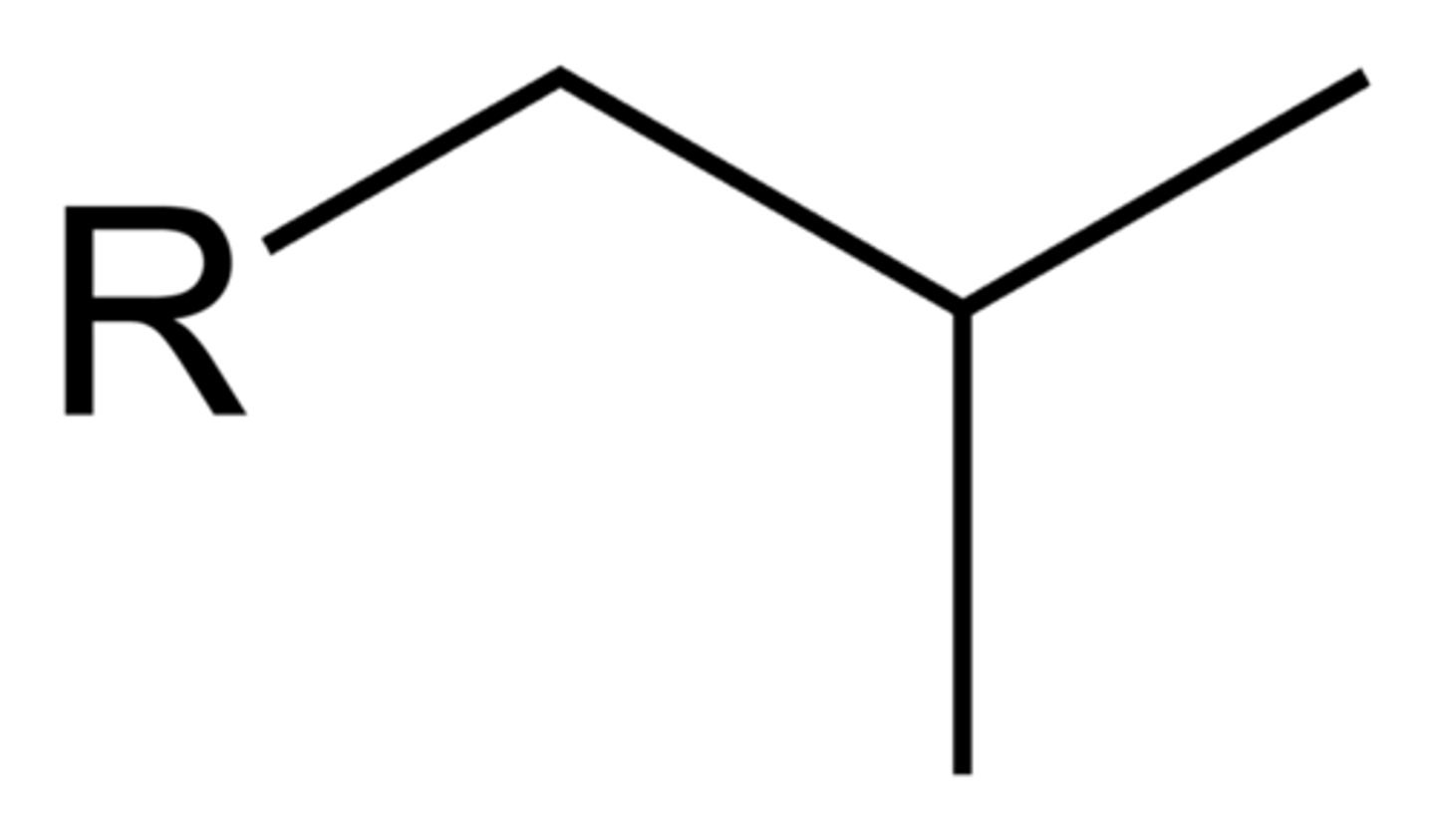
butyl
4 carbons
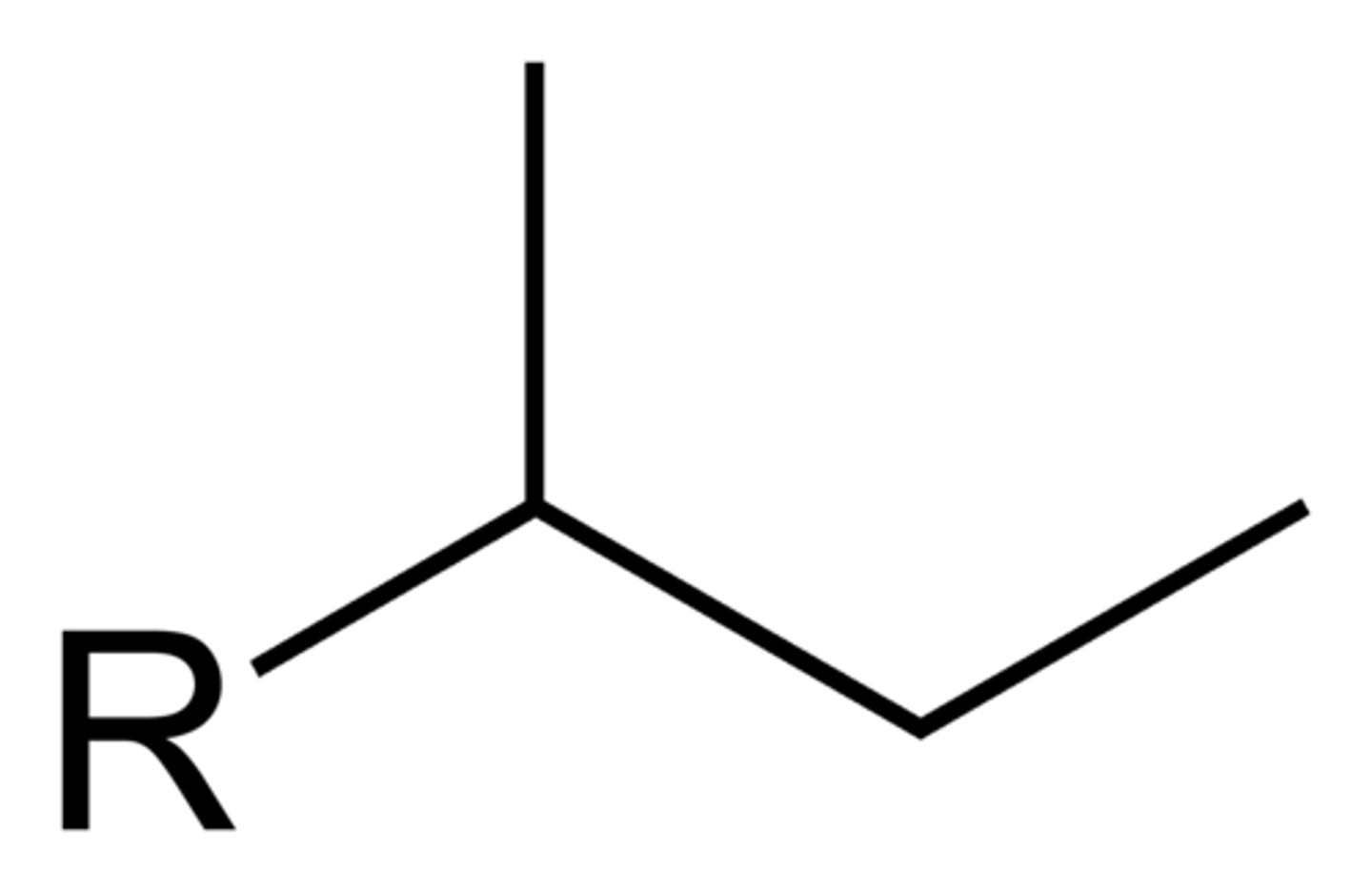
isobutyl
2-methylpropyl
sec-butyl
1-methylpropyl
tert-butyl
1,1-dimethylethyl
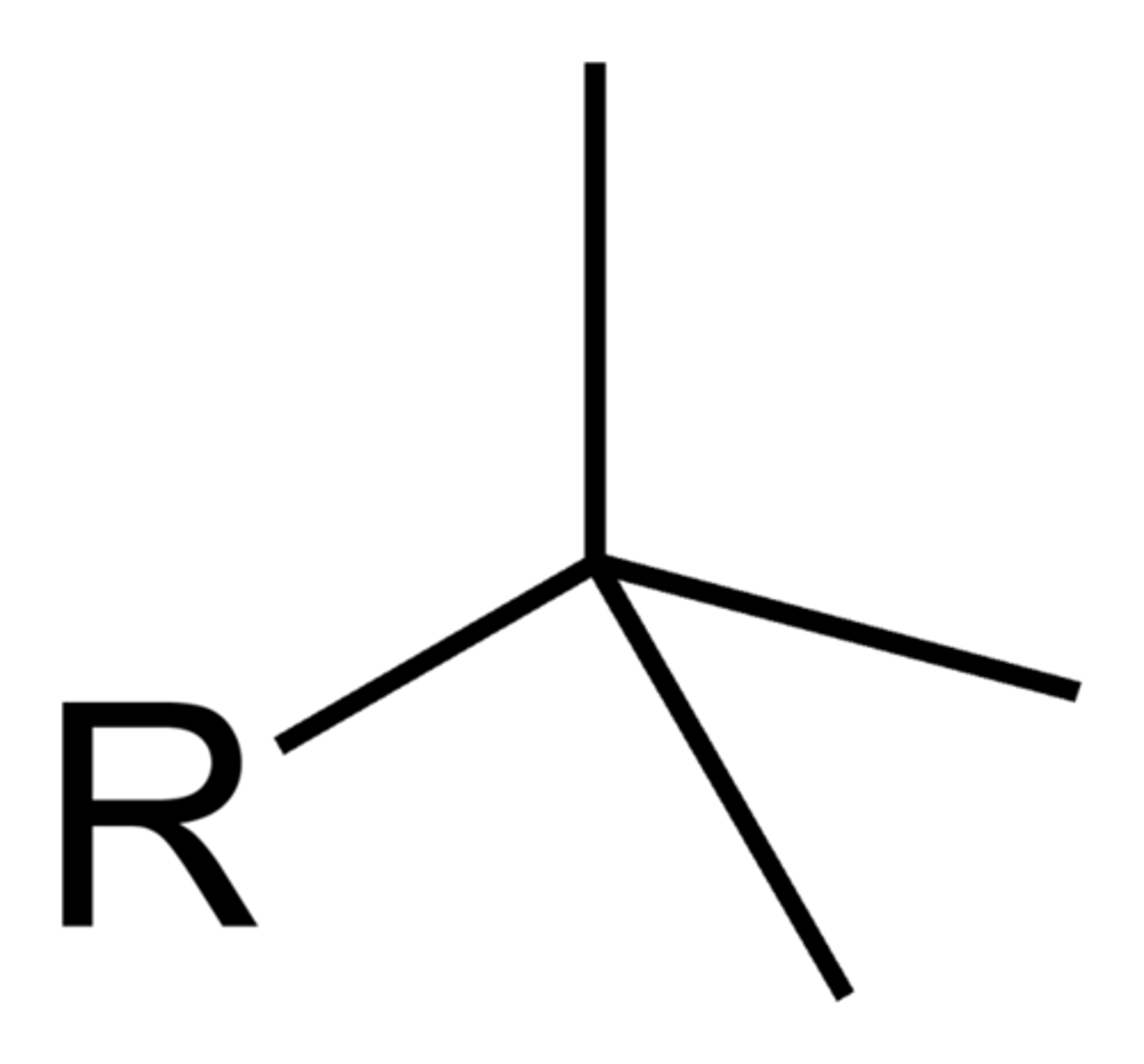
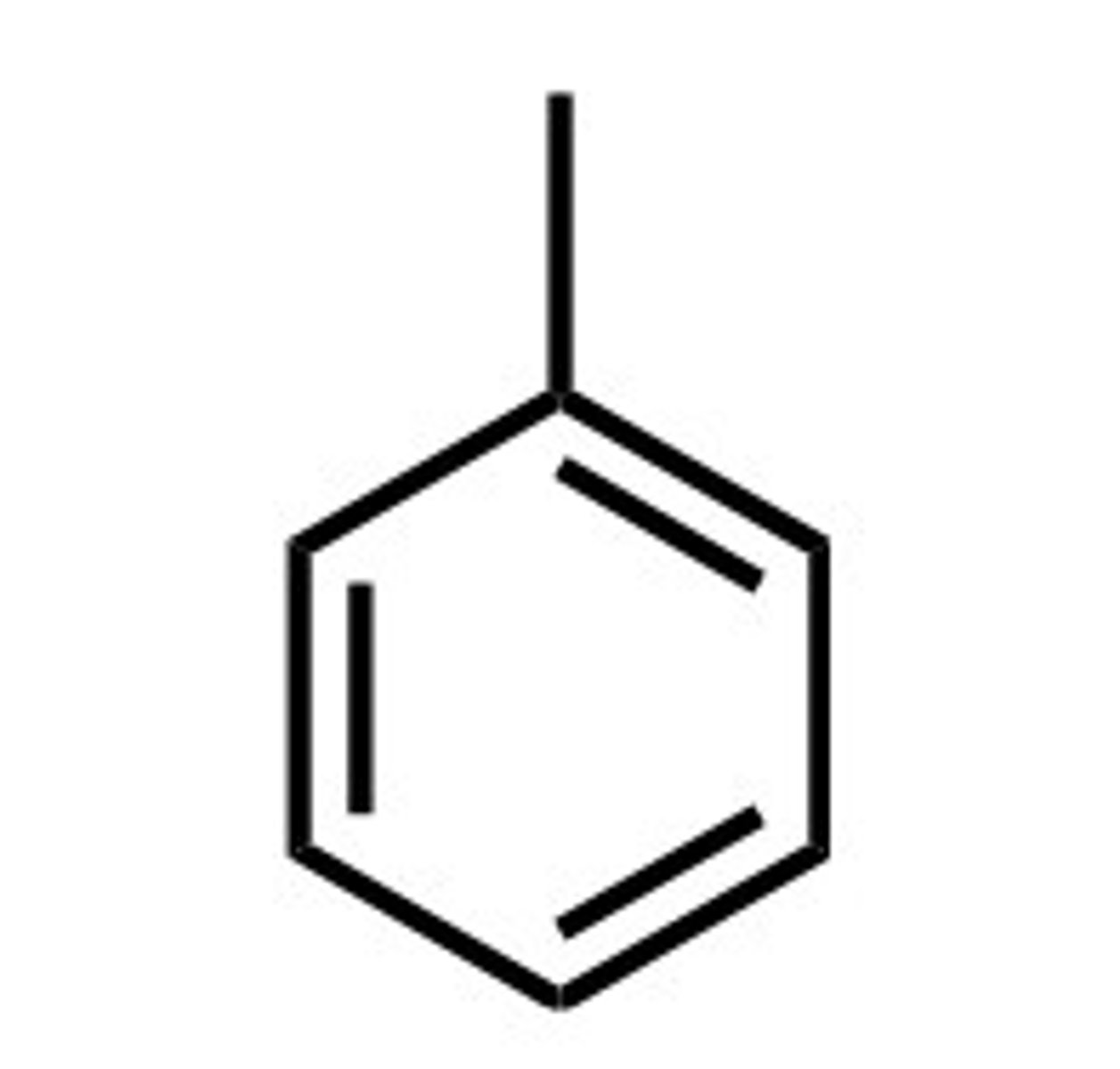
phenyl
benzene as a substituent
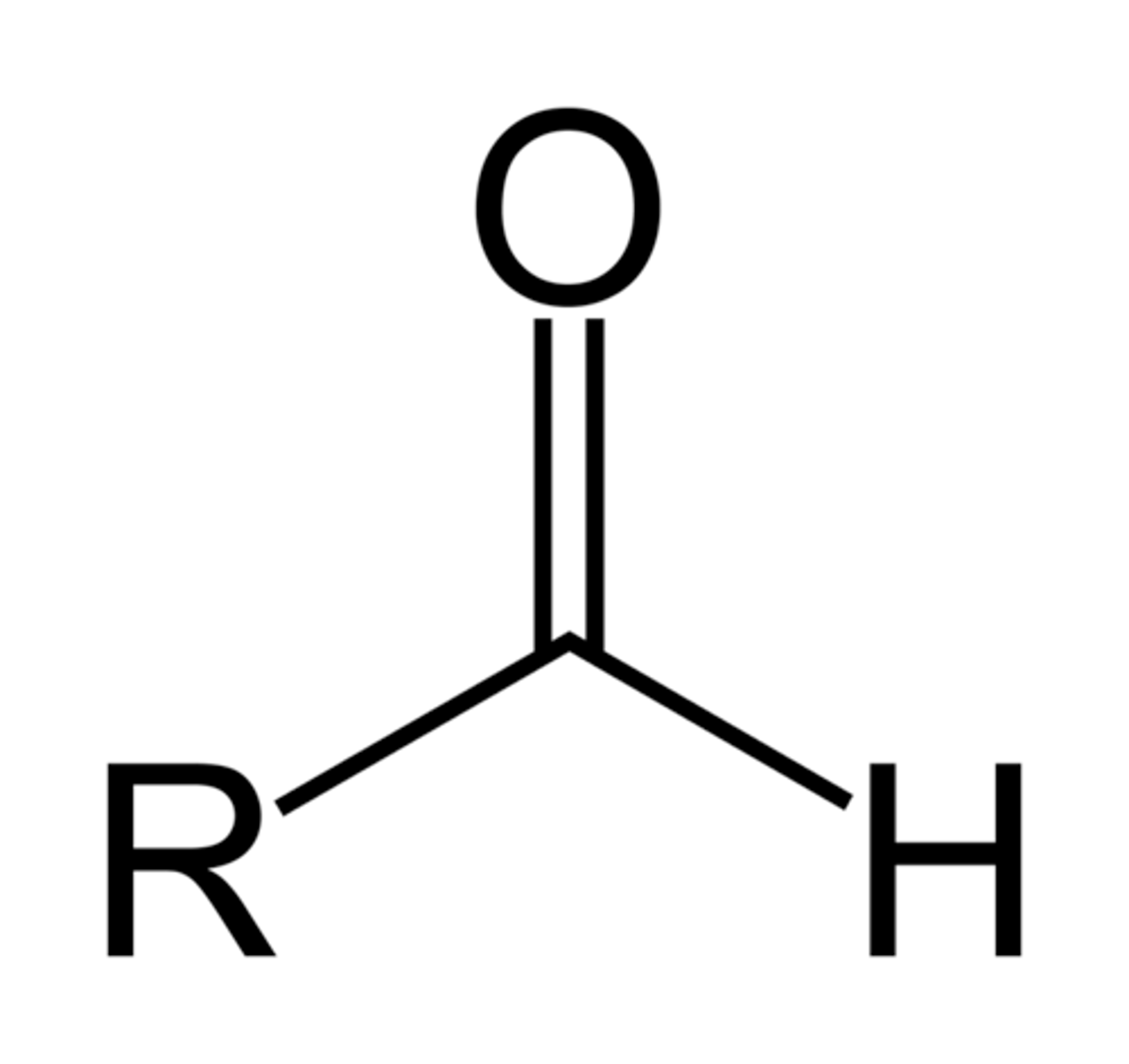
-al
double bonded O with an H
-ol
alcohol/OH
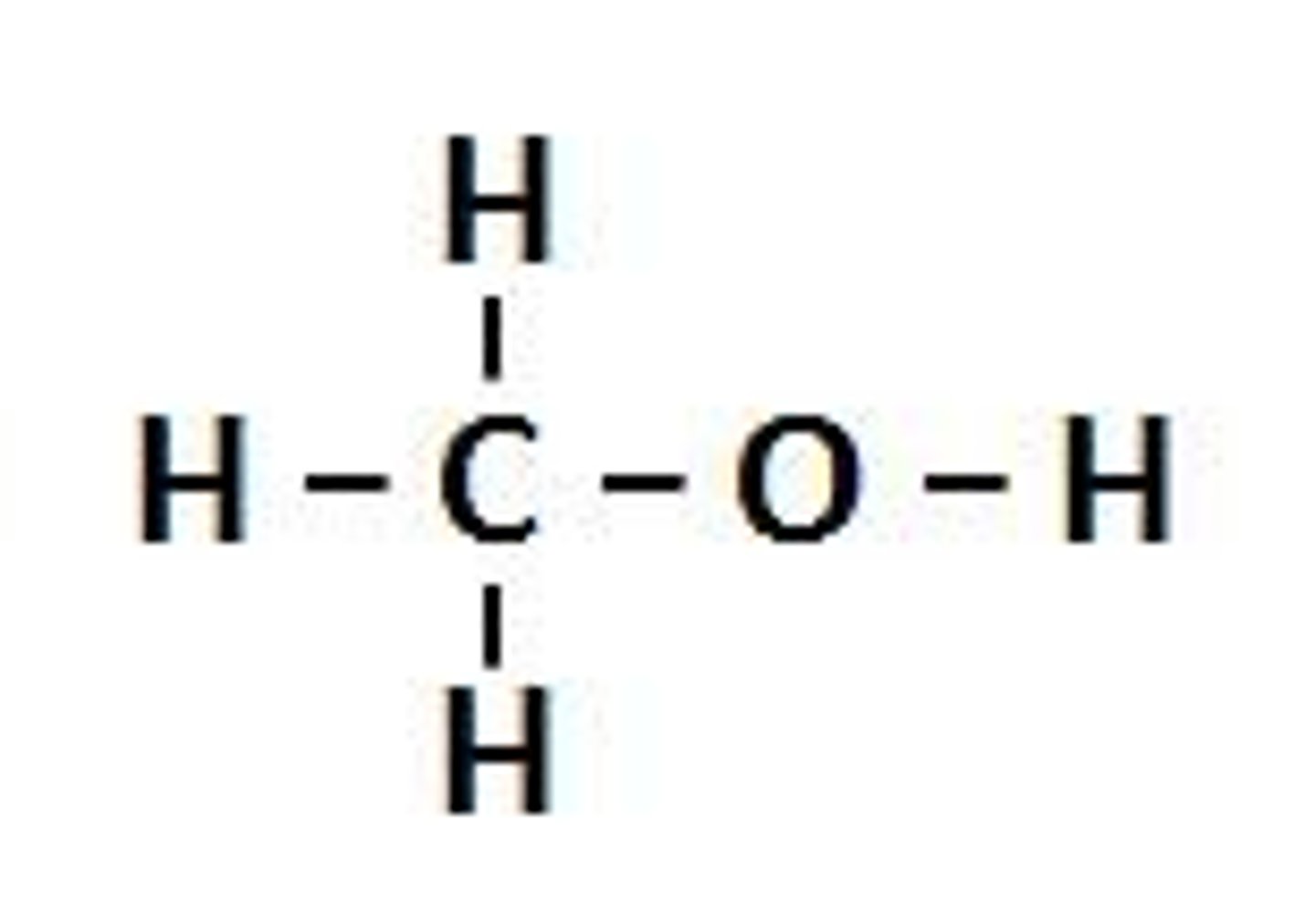
-ene
double bond
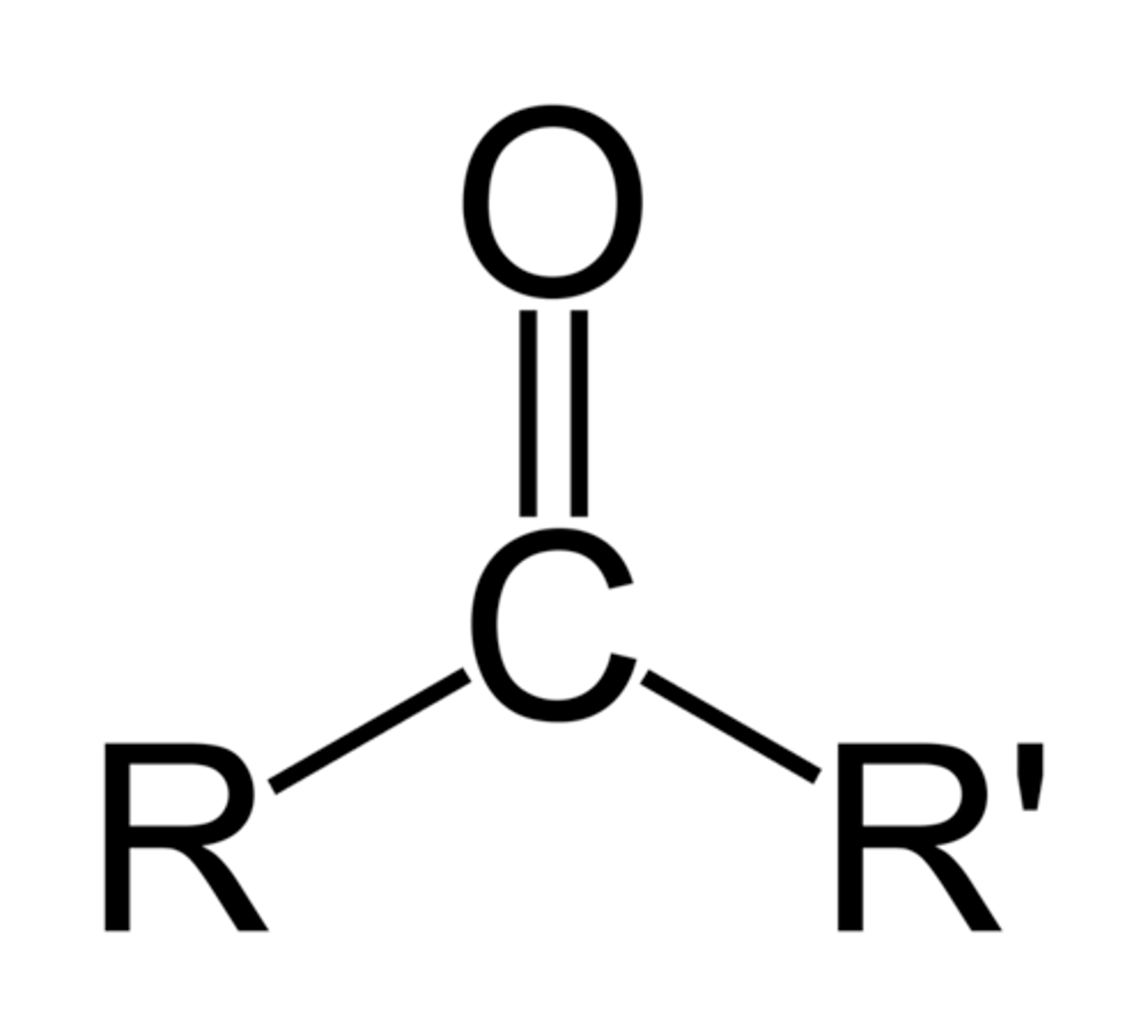
-one
double bonded O
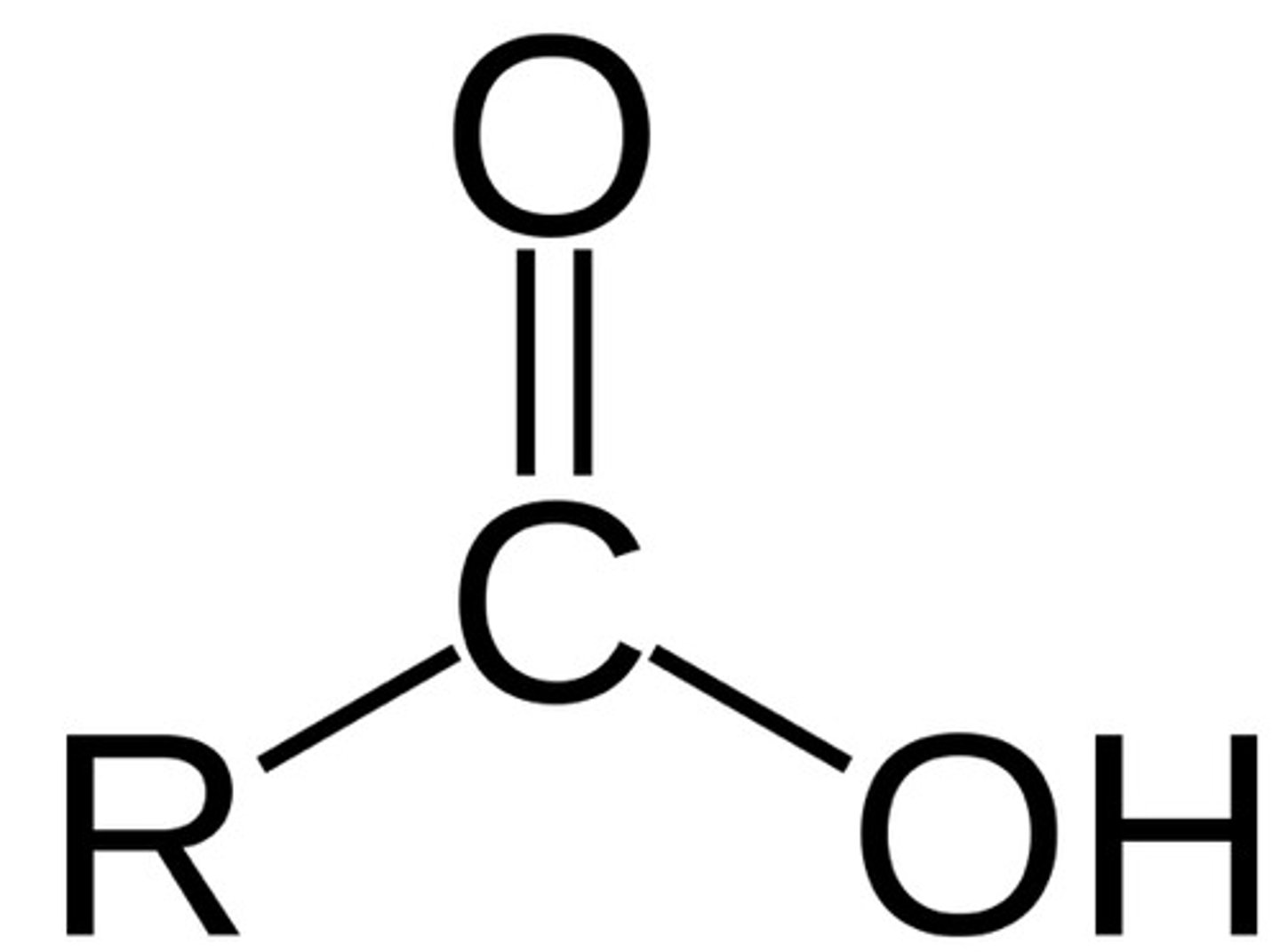
-oic
double bonded O with an OH
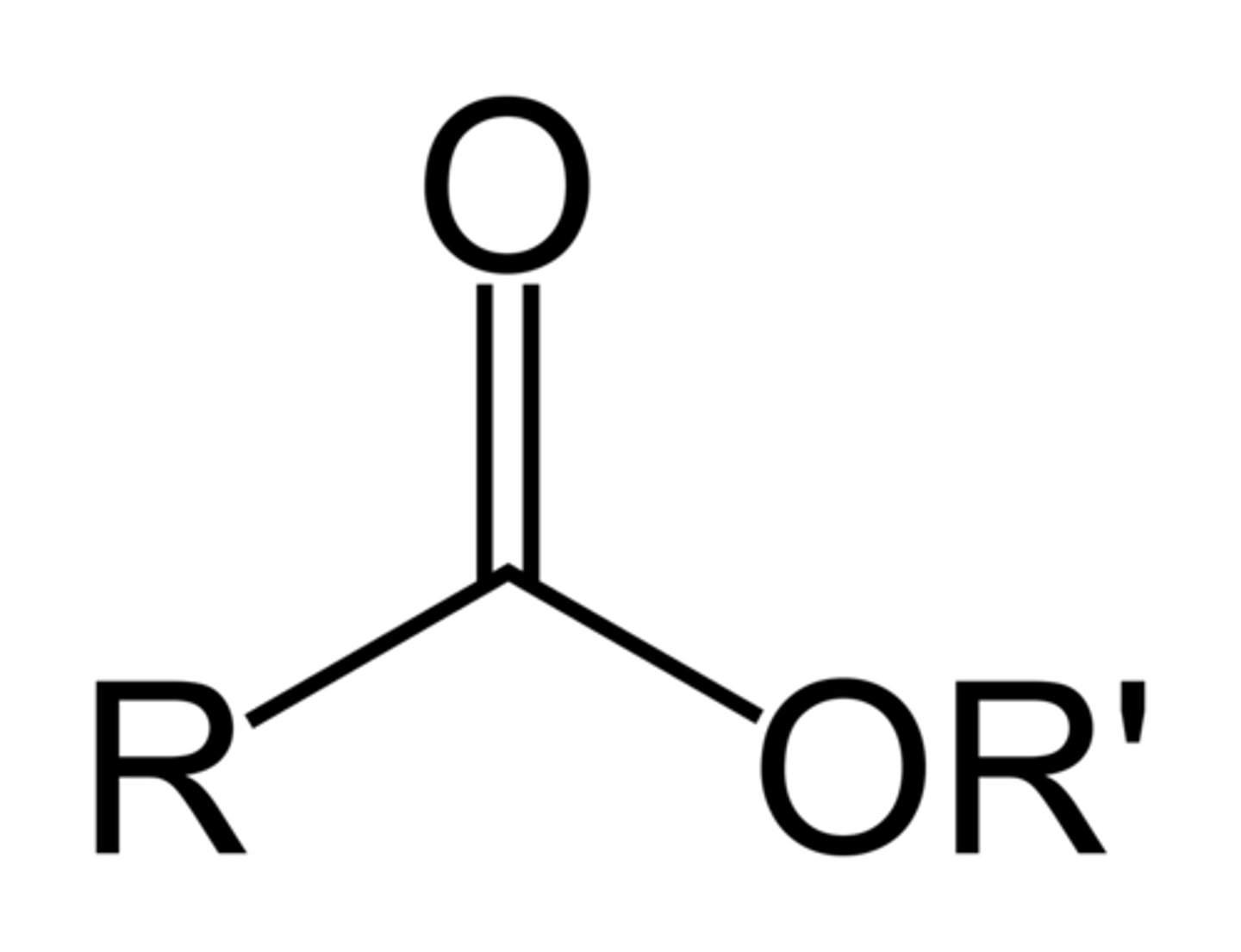
-oate
double bonded O with an O
sp3 hybridization
4 connections/tetrahedral/109.5
sp2 hybridization
3 connections/trigonal planar/120
sp hybridization
2 conections/linear/180
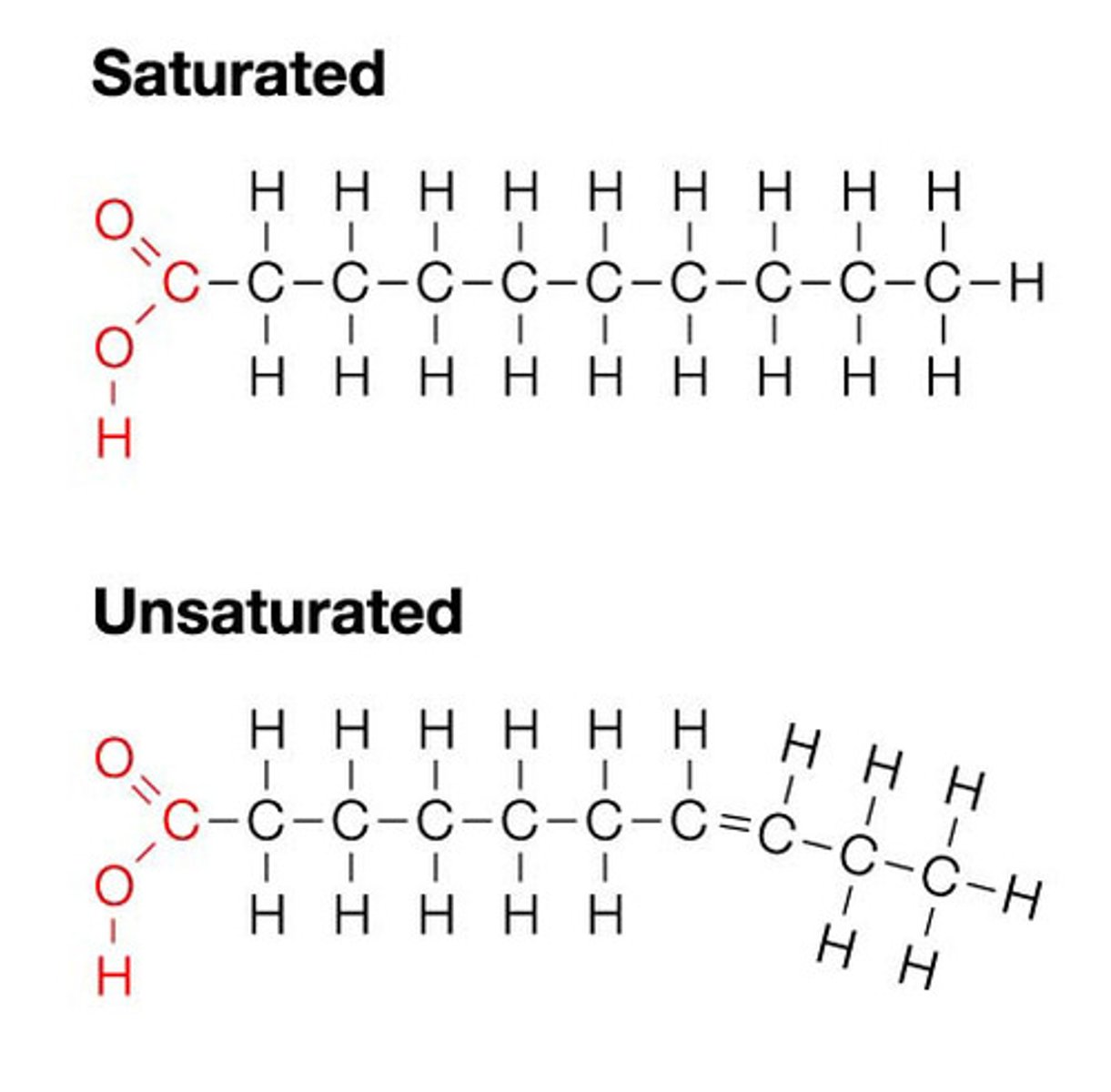
lipids
long structure
Carbohydrates
ring w/o N
amino acids and proteins
contains N,O,C,H
nucleic acids (DNA and RNA)
has to have phosphorus/nucleotide base have nitrogen rings
Huckels Rule
4n+2=x
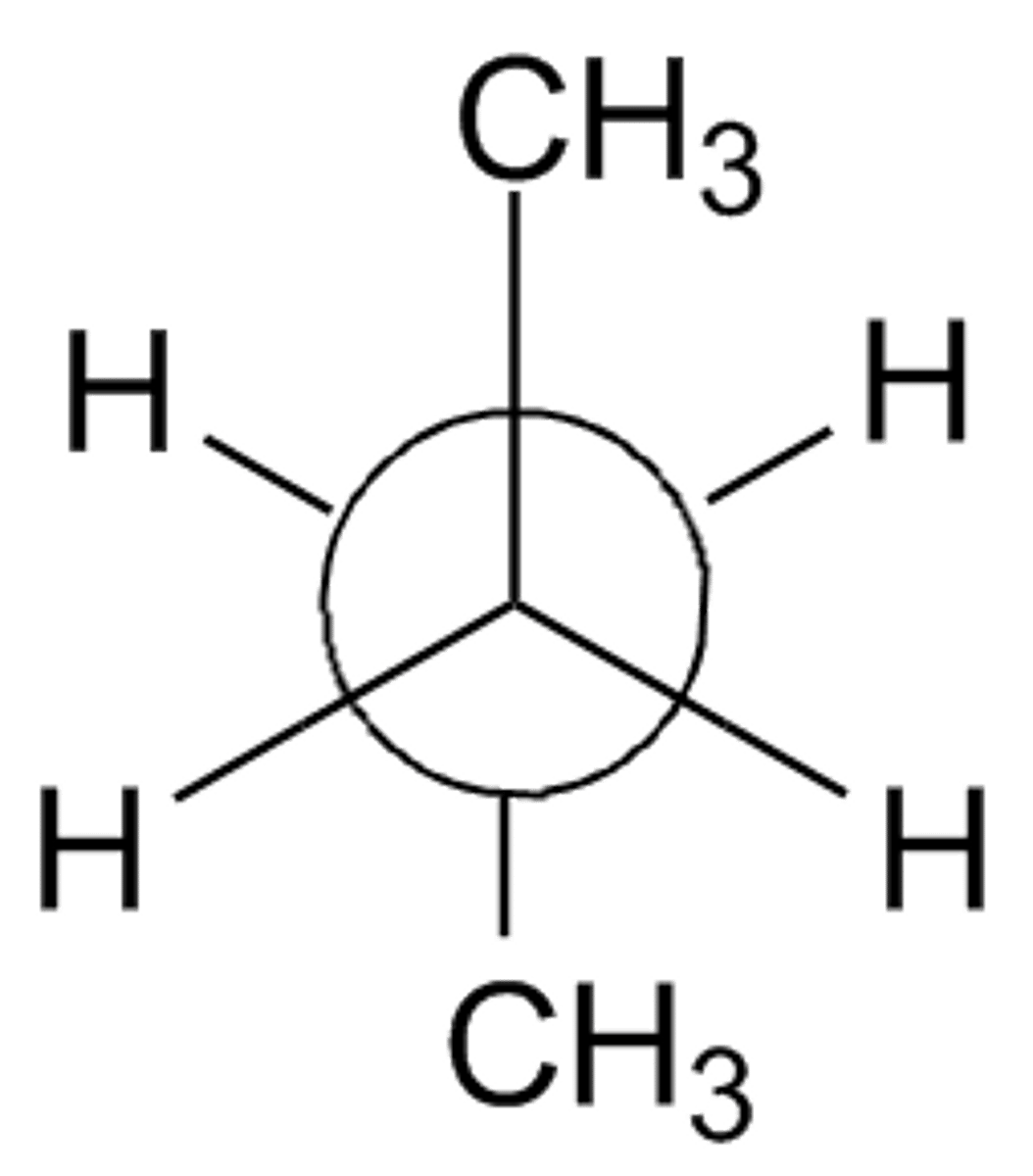
staggered conformation
more stable
less energy
eclipsed conformation
less stable
more energy
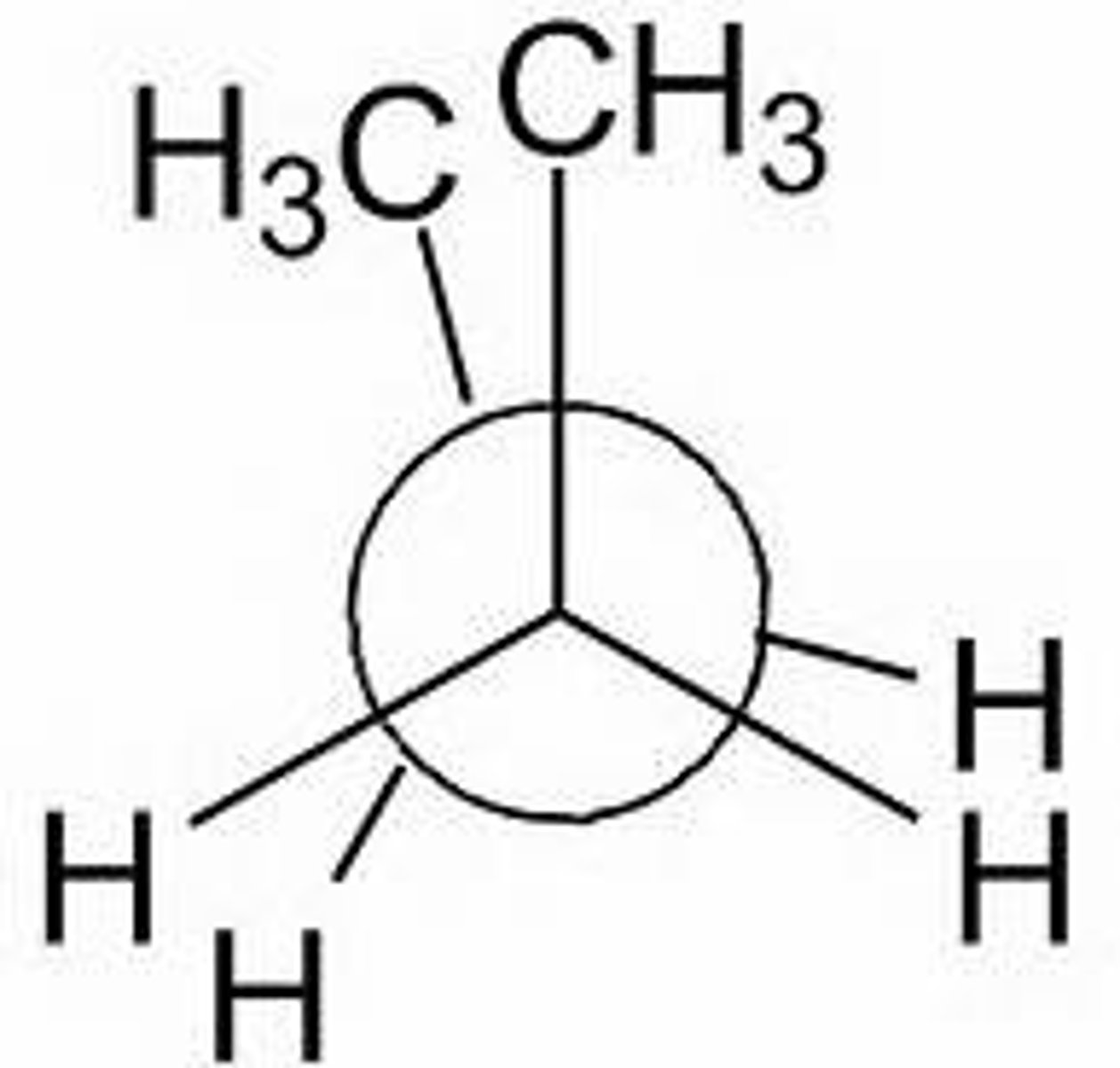
Angle for eclipse
0
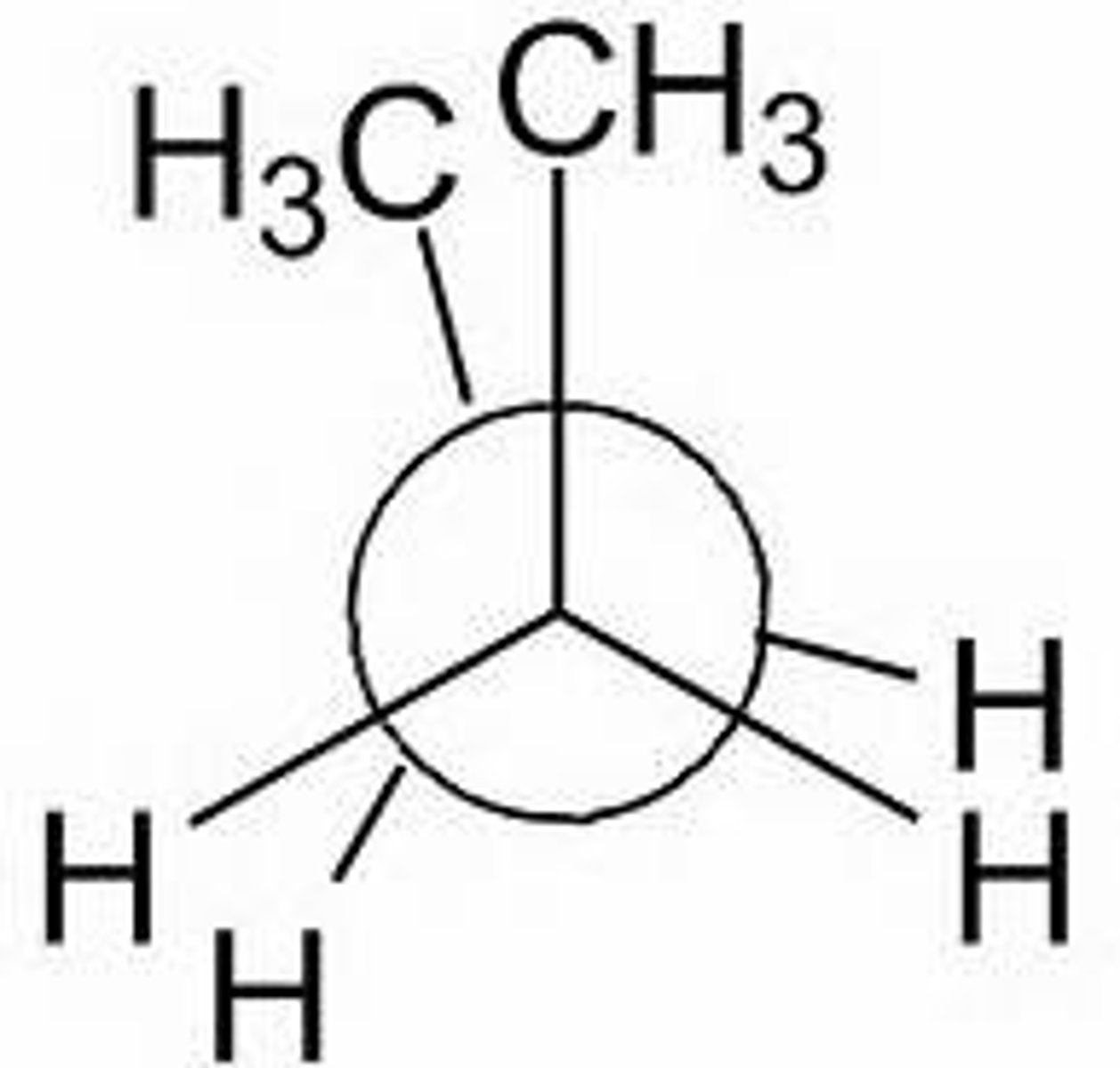
Anti angle for staggered
180
gauche angle for staggered
60
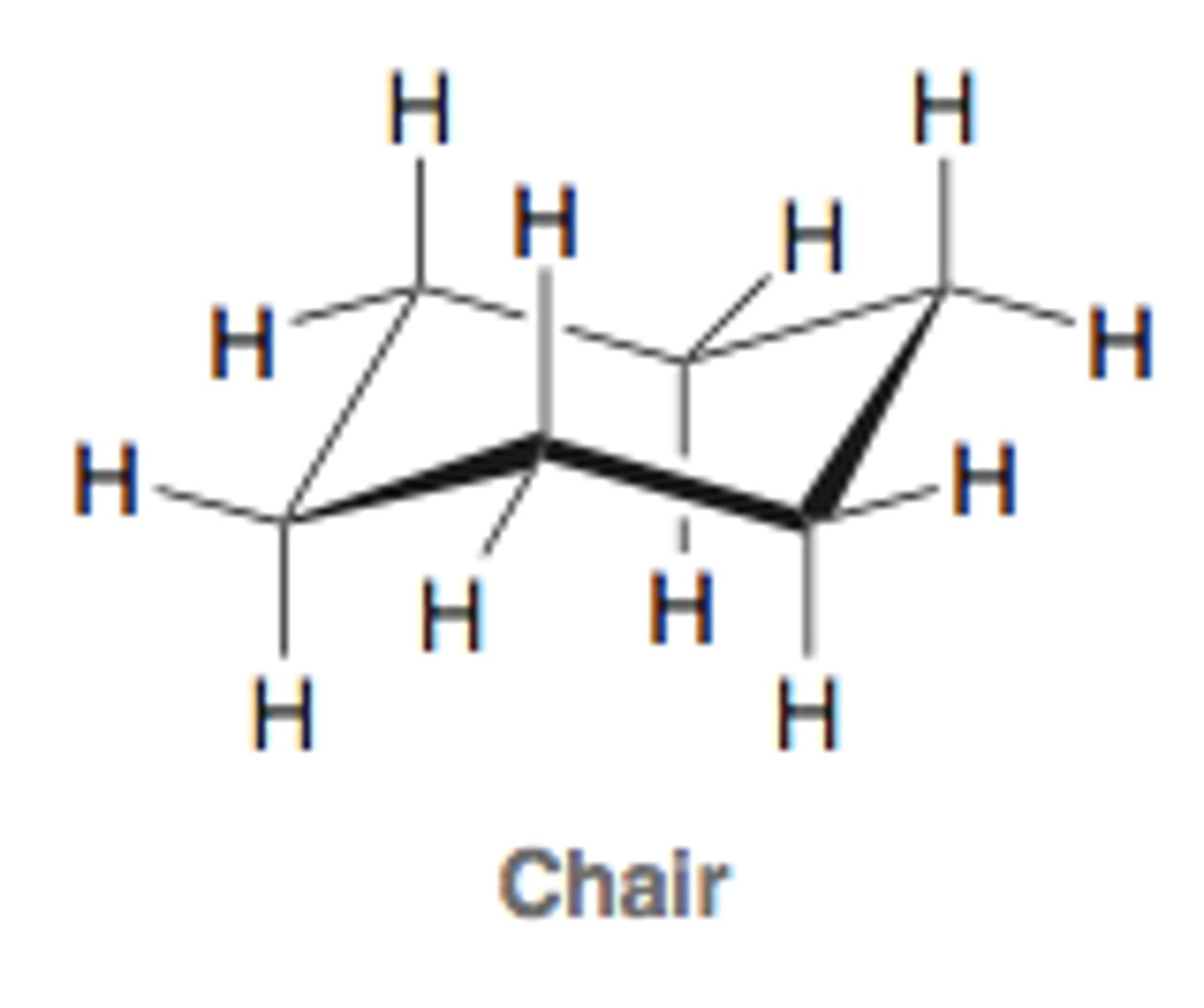
chair conformation
most stable conformation of cyclohexane
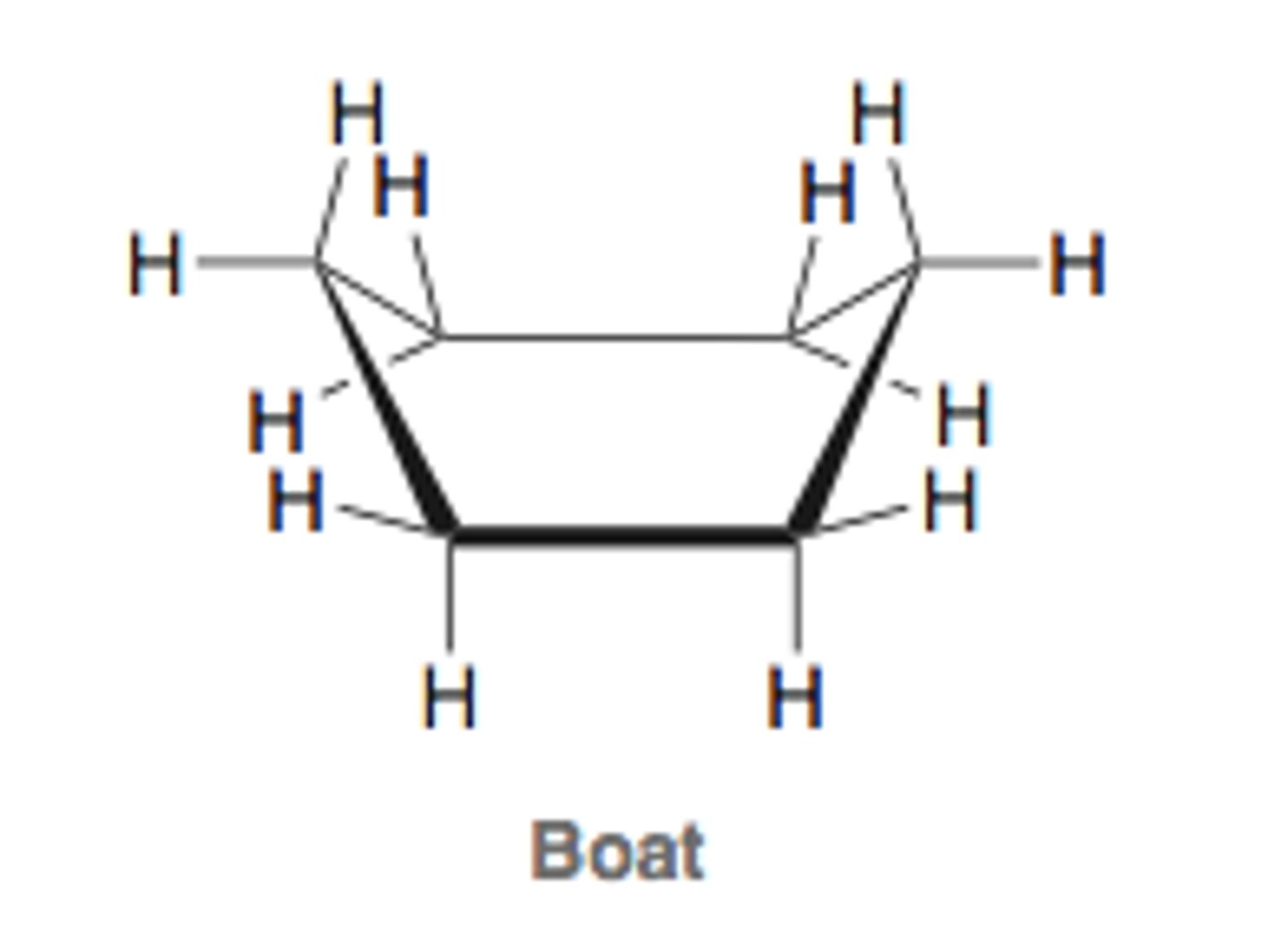
boat conformation
high energy
less stable conformation of cyclohexane
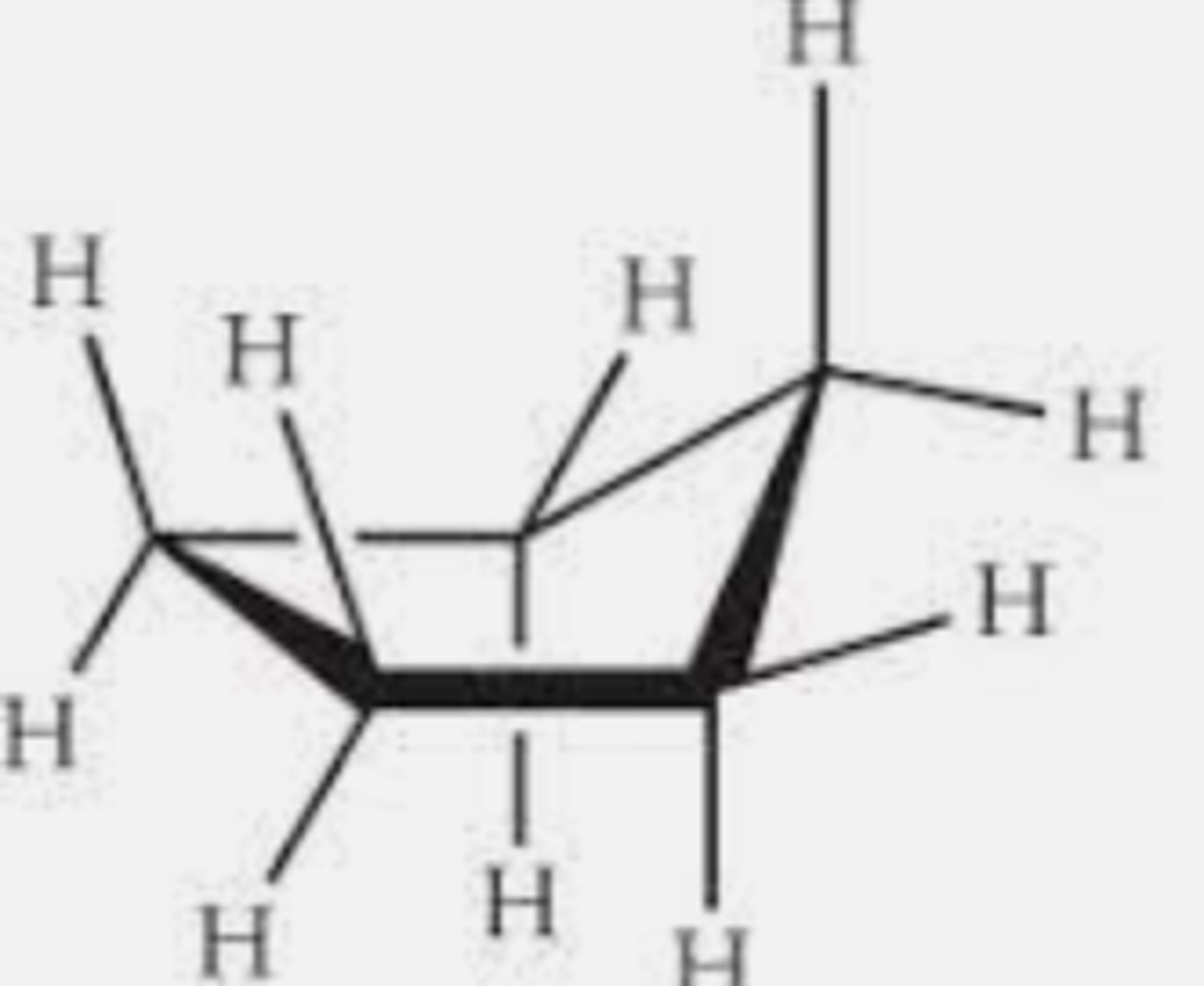
Envelope conformation
most stable conformation of cyclopentane
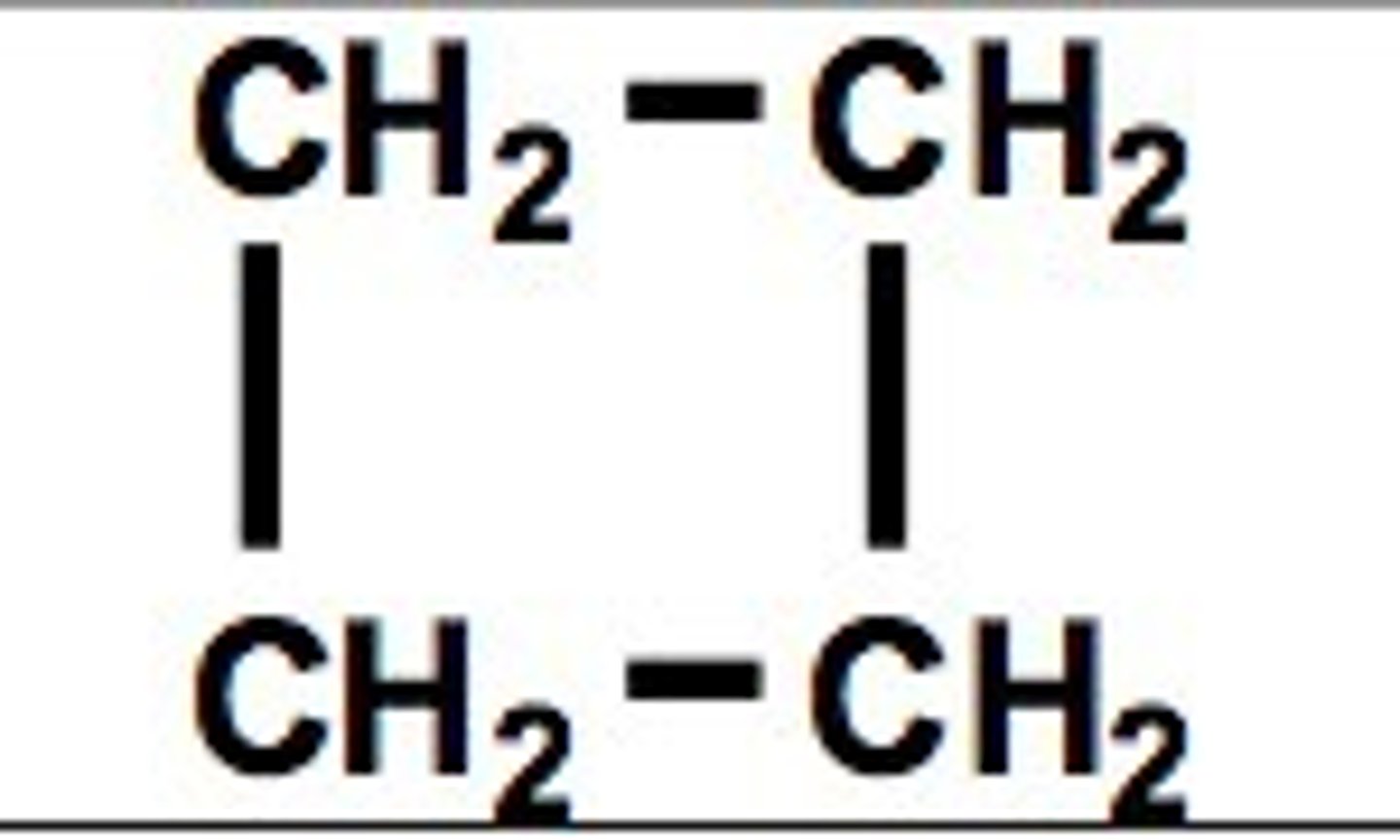
cyclobutane
C4H8
Square
cyclopropane
triangle
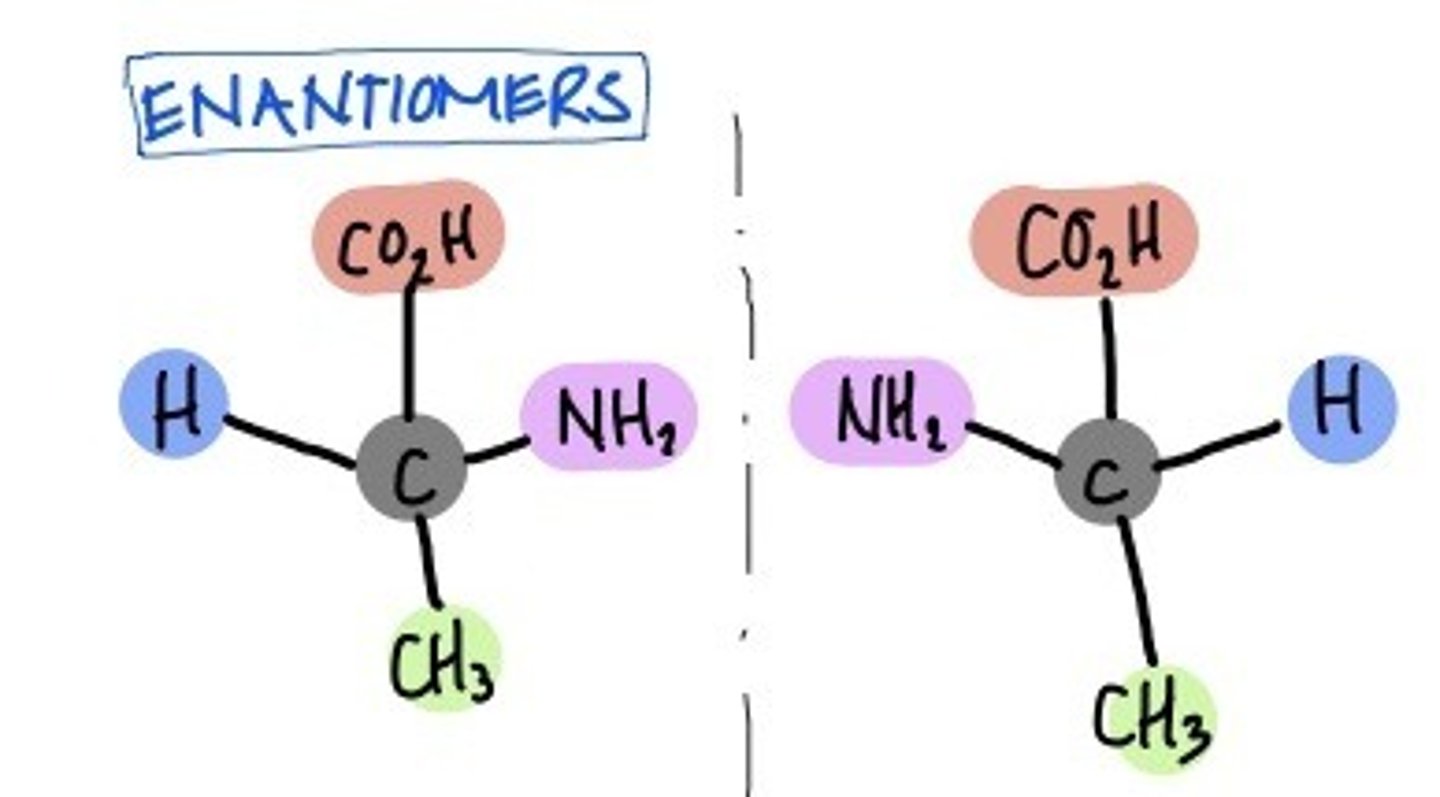
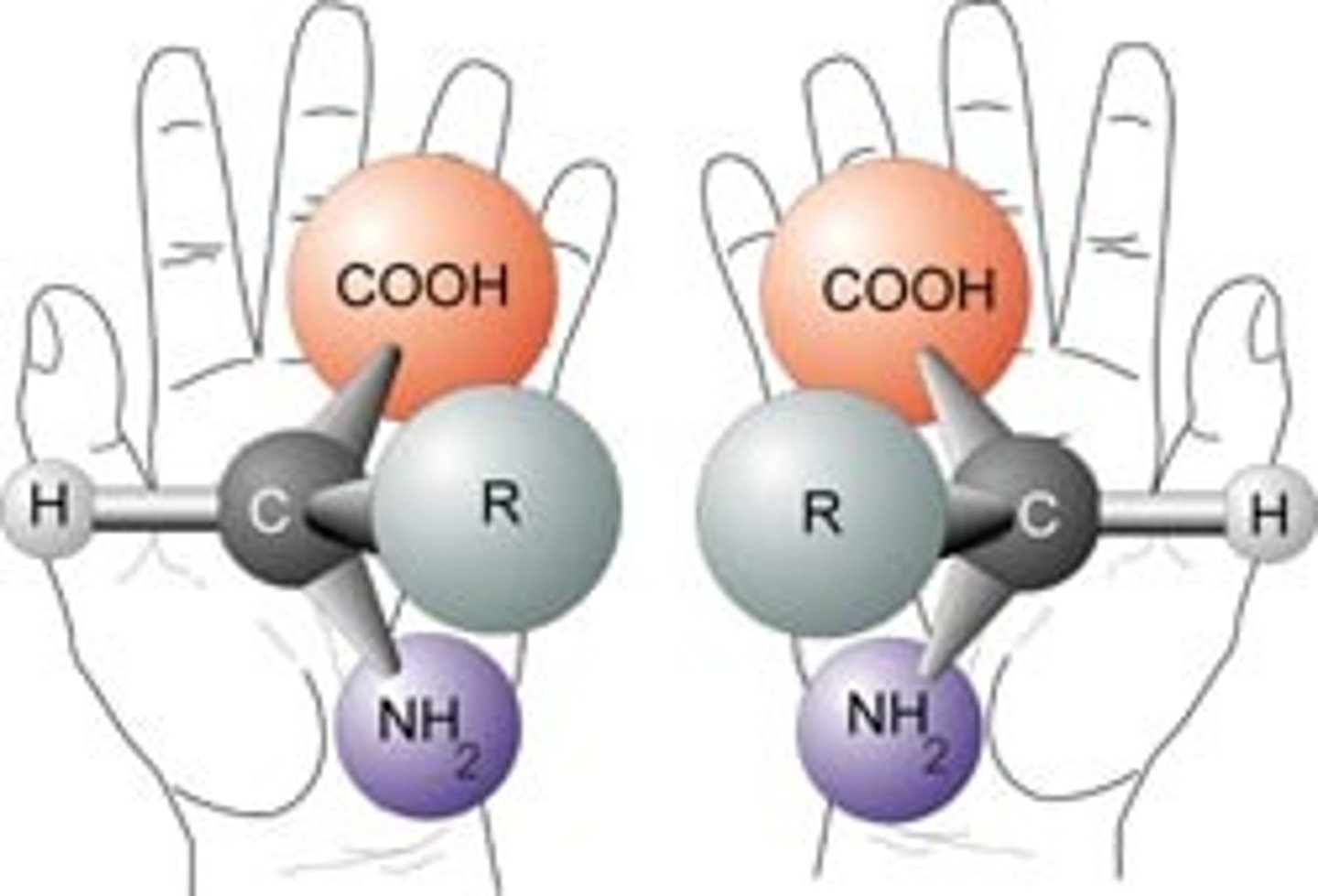
Enantionmers
isomers that are mirror images of each other and non-superimposable
"these two molecules are enantiomers of each other"
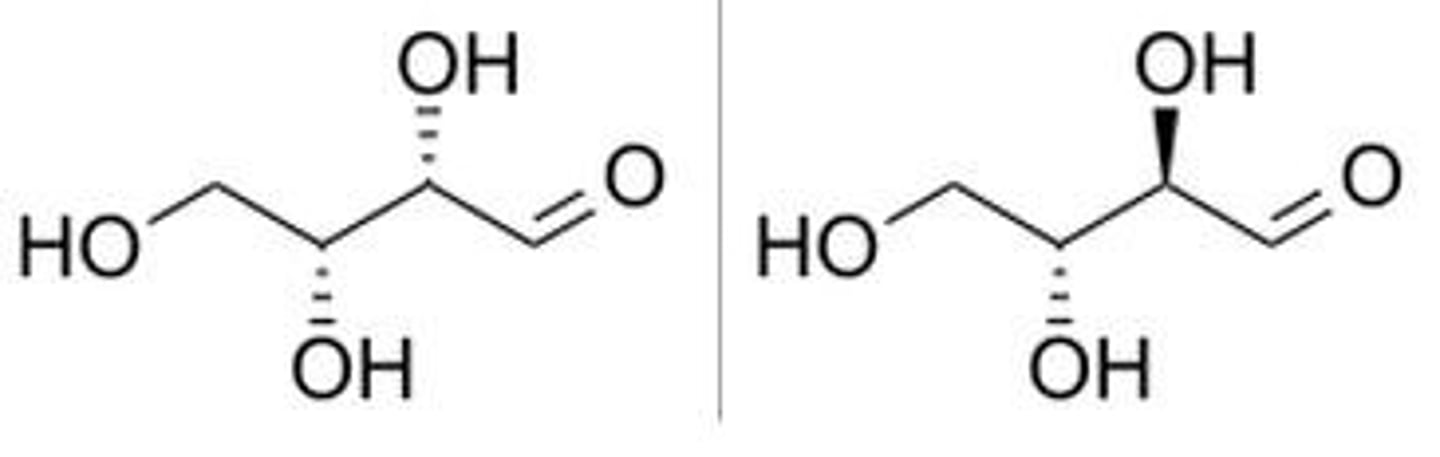
diastereomers
stereoisomers that are not mirror images and non-superimposable
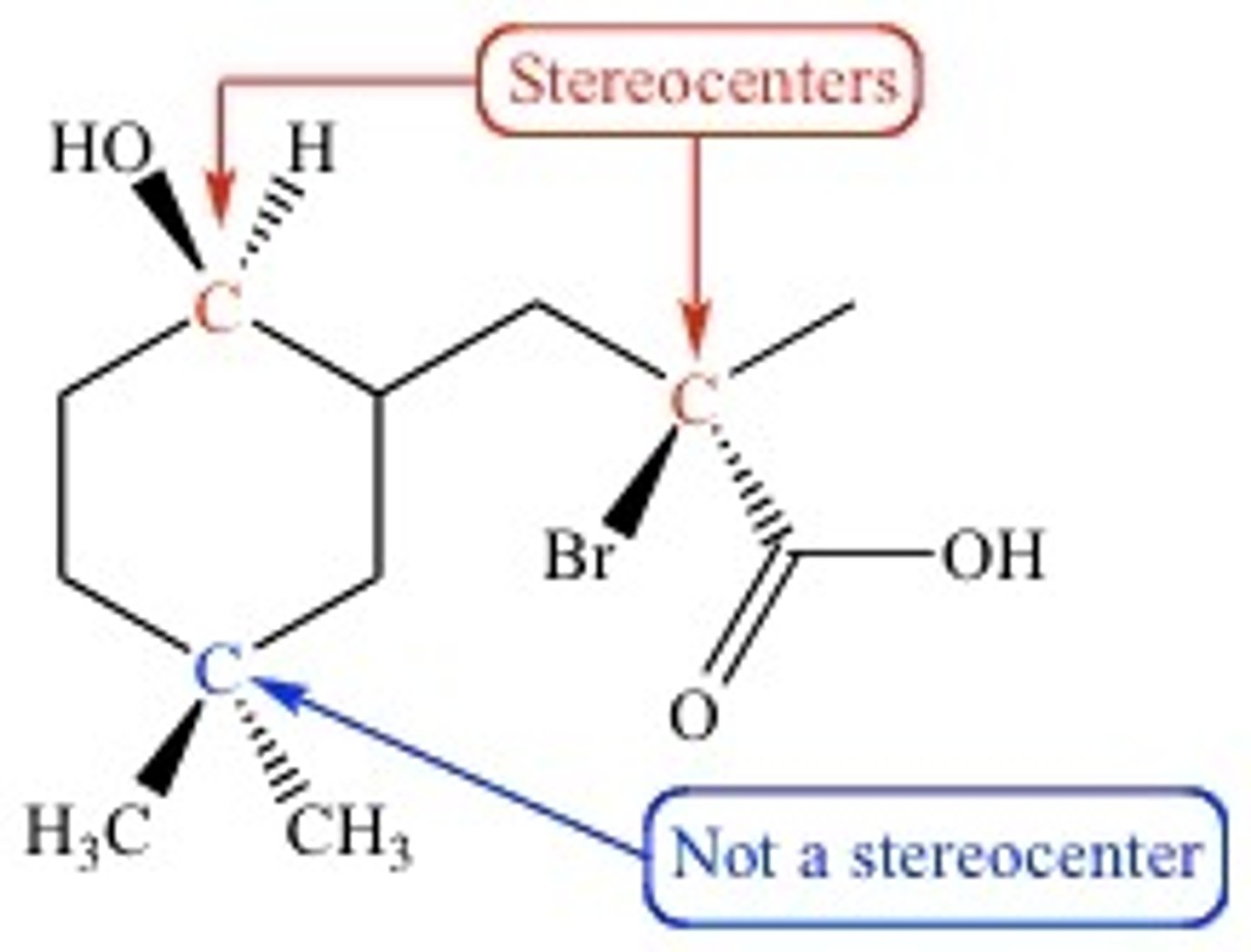
stereocenter
sp3 atoms that have 4 chemically inequivalent substituents
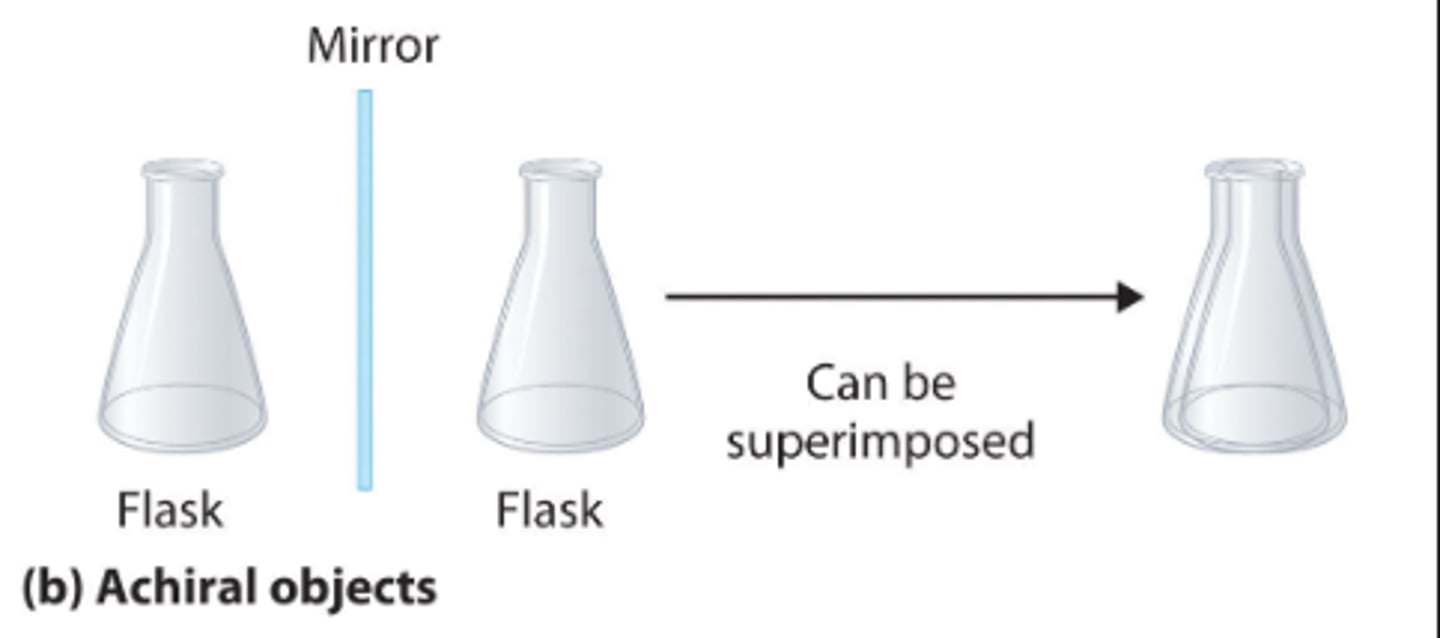
achiral
A molecule that is superimposable/have a plane of symmetry on its mirror image
optically inactive
Stereisomers
structures that have the same MF, same connectivity of atoms, but different spatial geometry
Chiral
a molecule that is not superimposable on its mirror image
"this molecule is chiral"
optically active
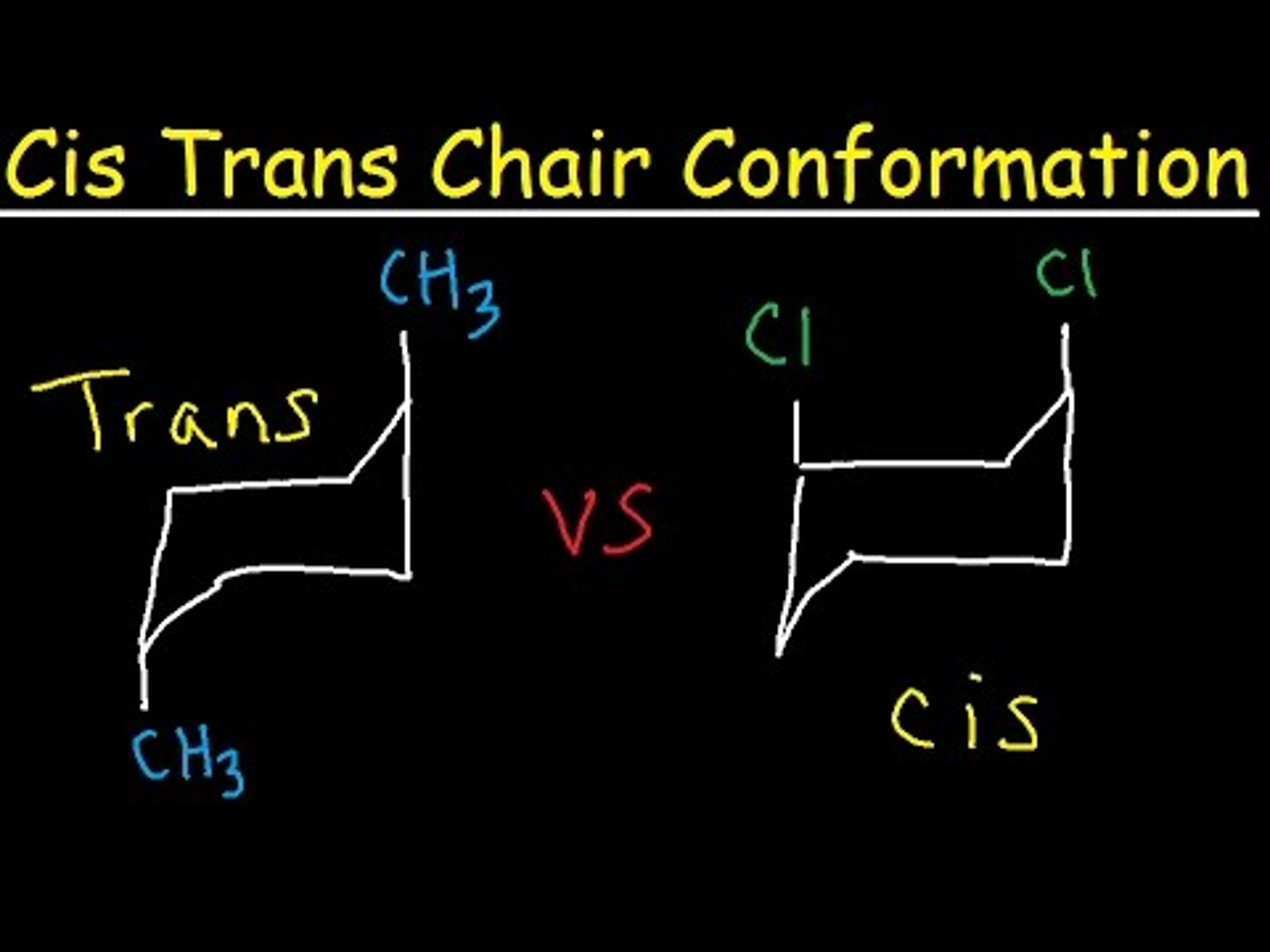
trans
ex: one H is above the ring and one is below
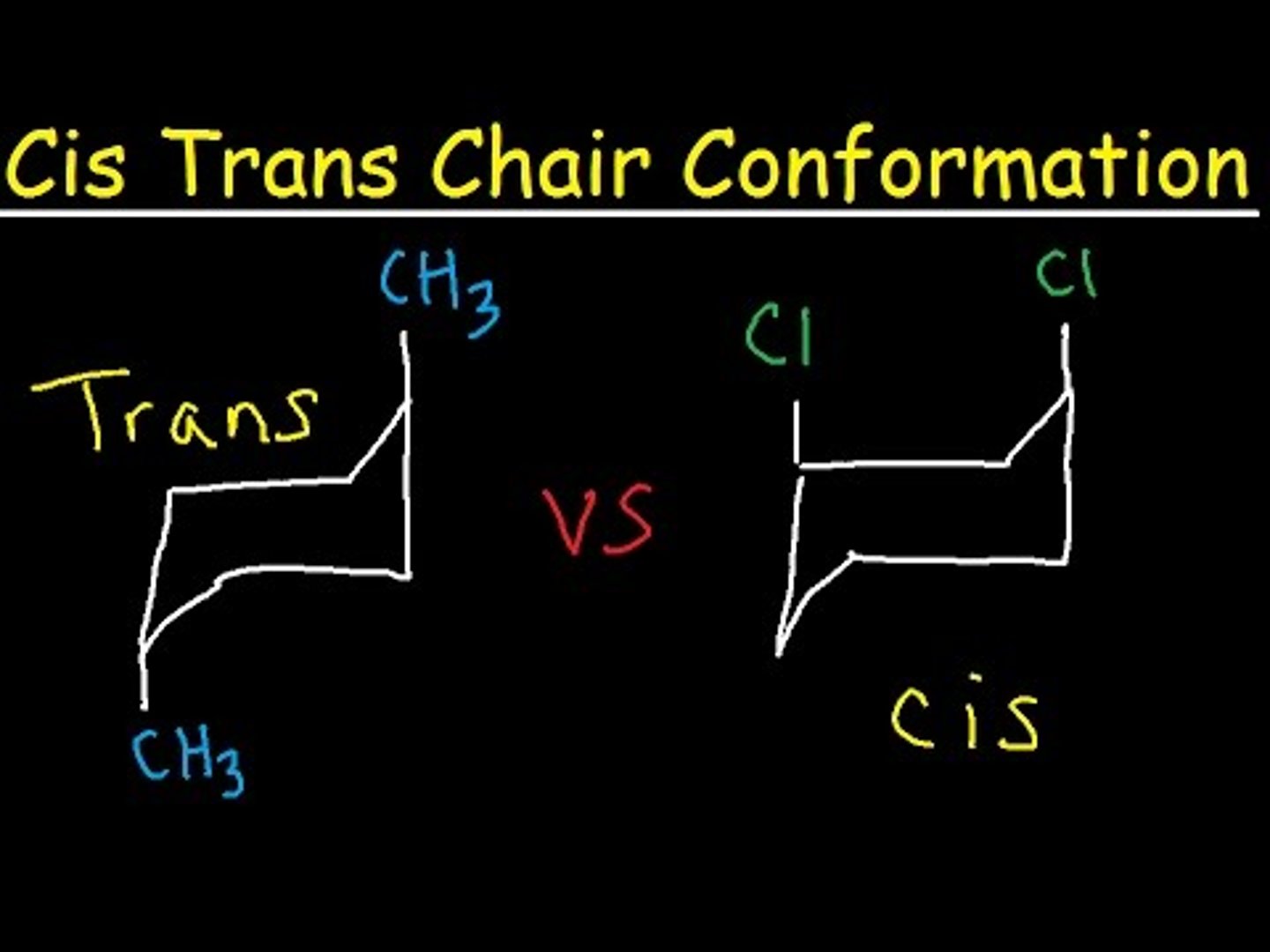
cis
ex: both H's are above the ring or both H's are below the ring
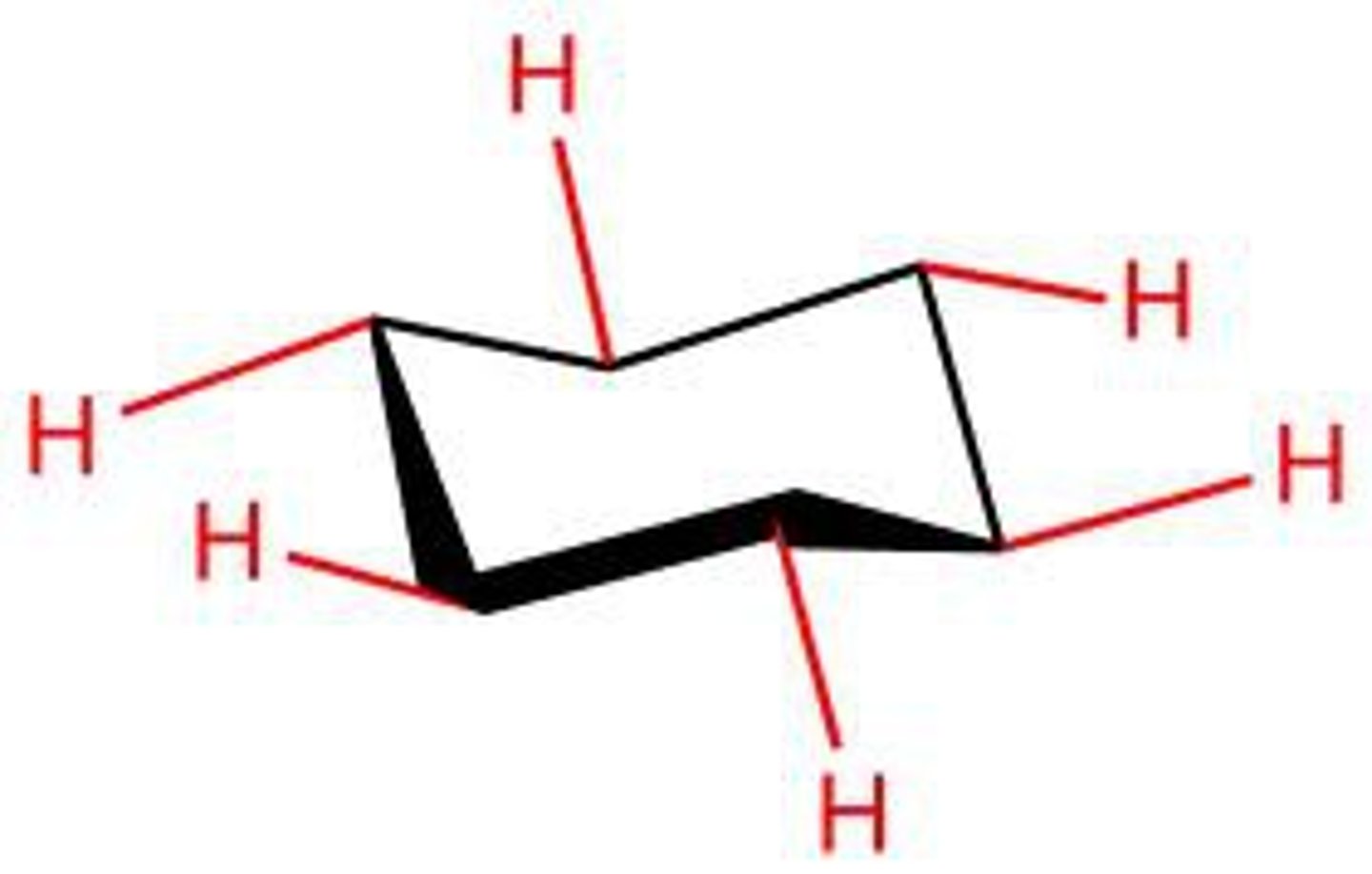
axial
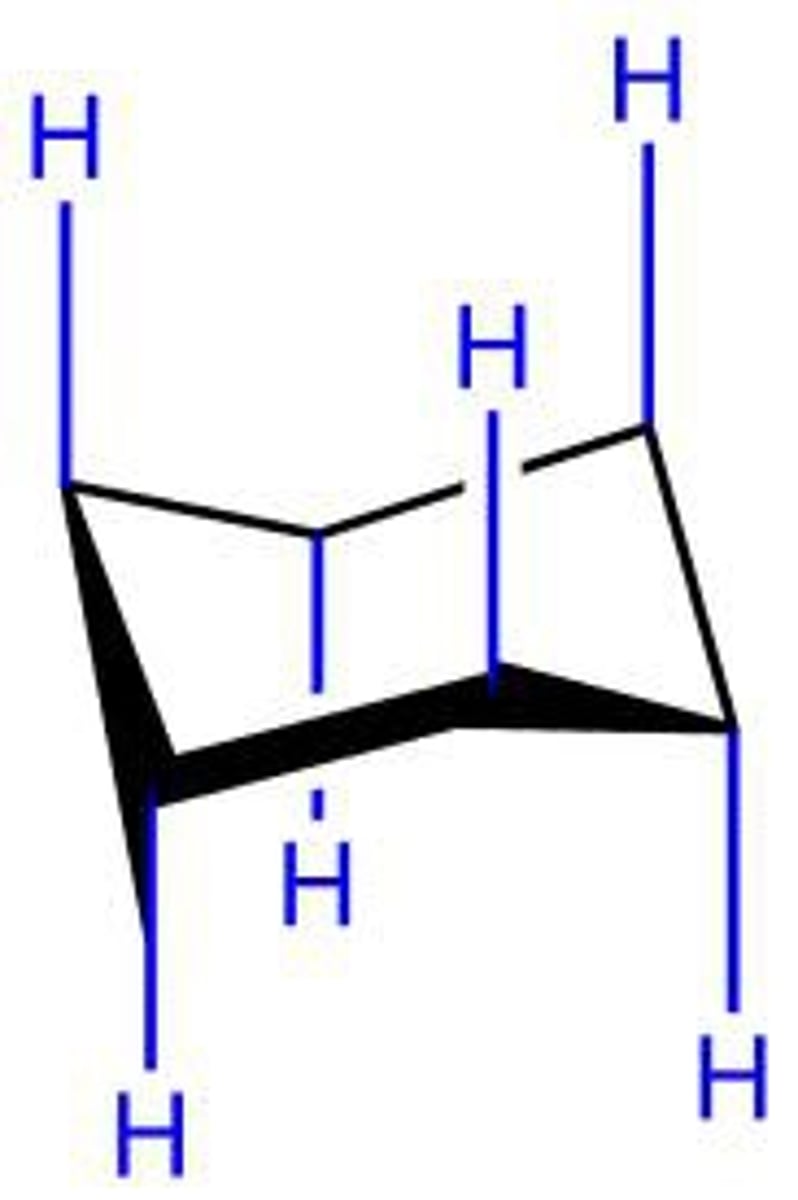
equatorial
diaxial interactions
interactions between groups in parallel axial positions on the same side of a chair conformation of a cyclohexane ring
dashes
away from you
down in chair
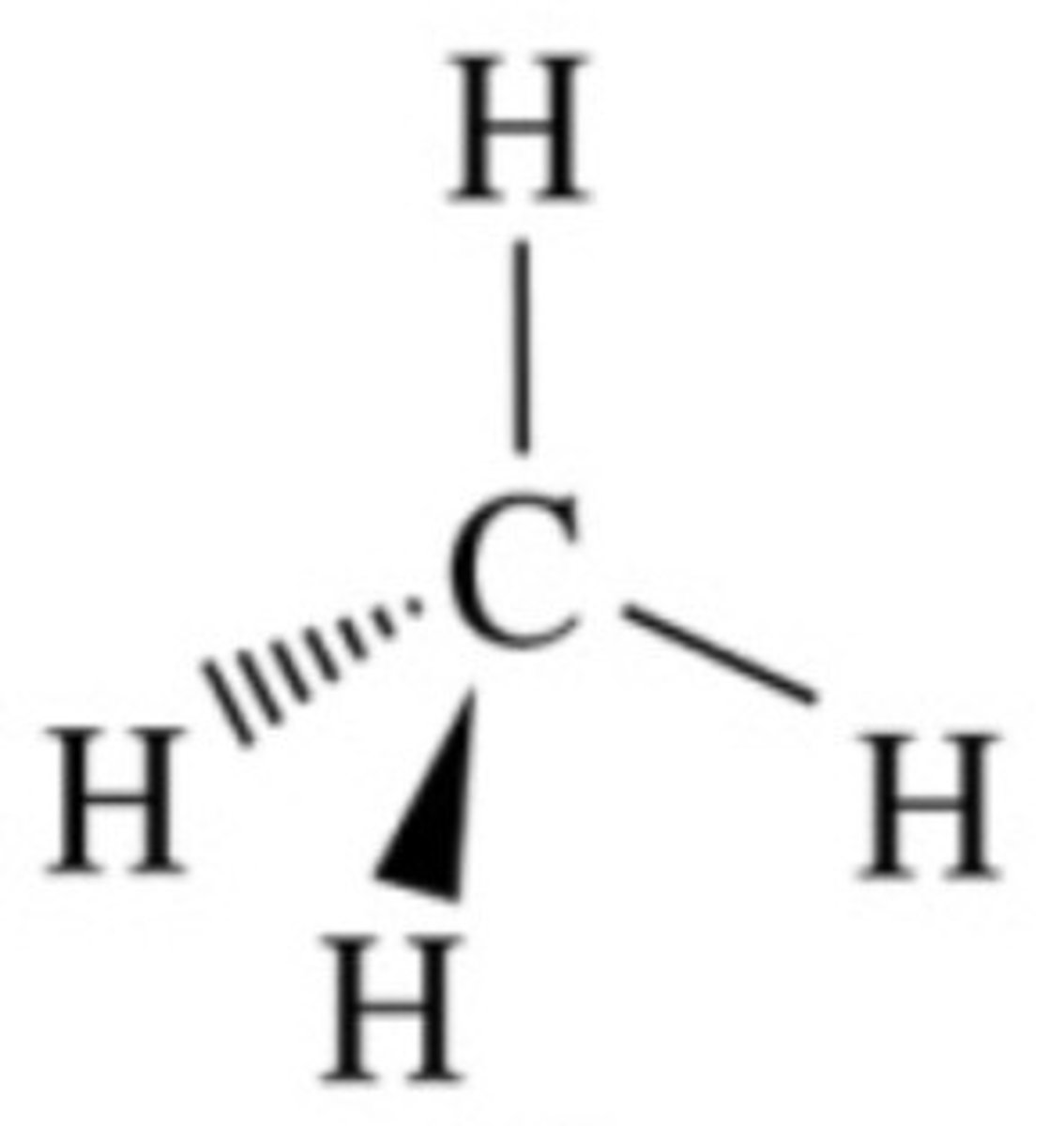
wedges
coming at you
up in chair
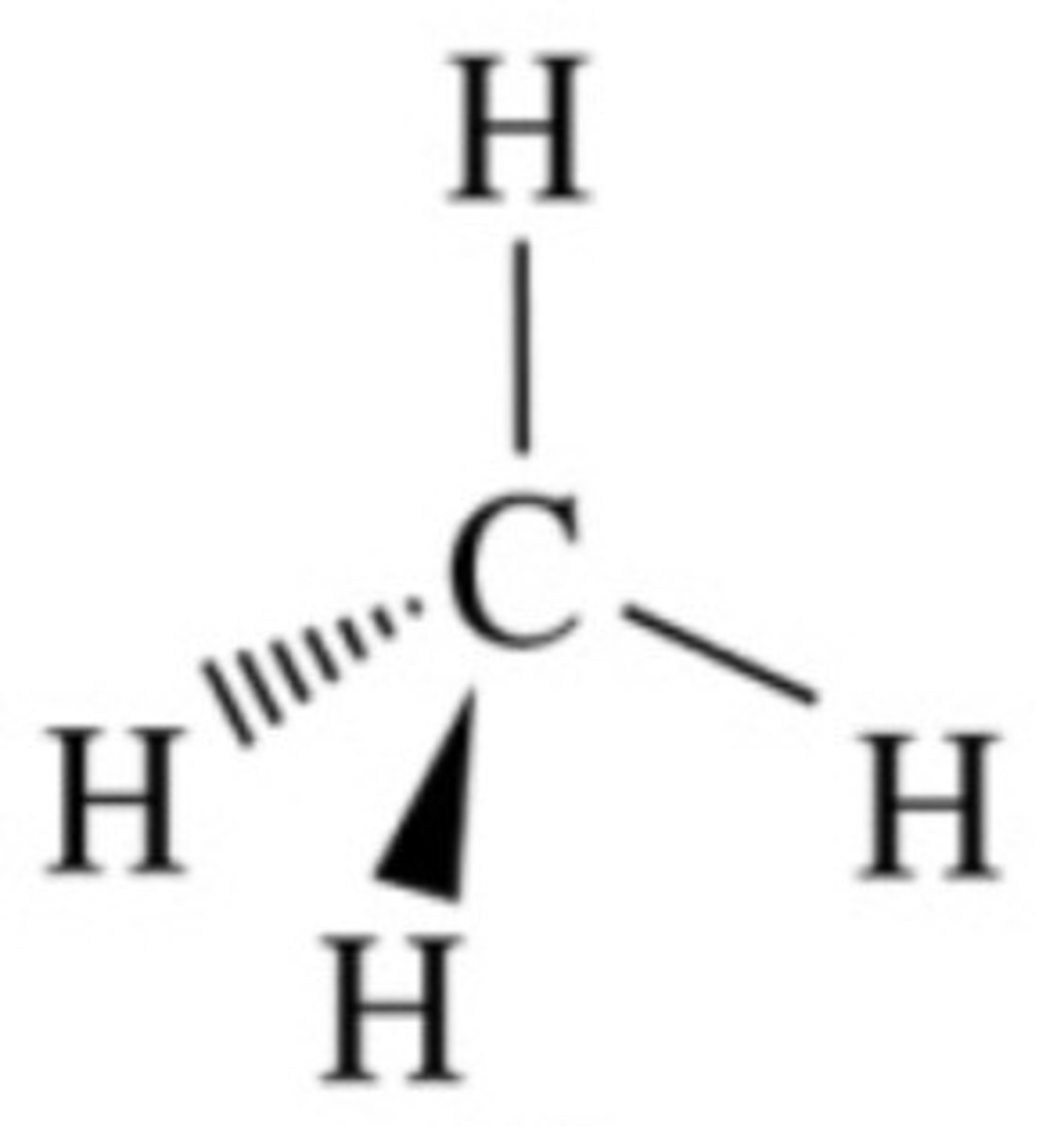
-yne
triple bond
-ane
single bond
propane
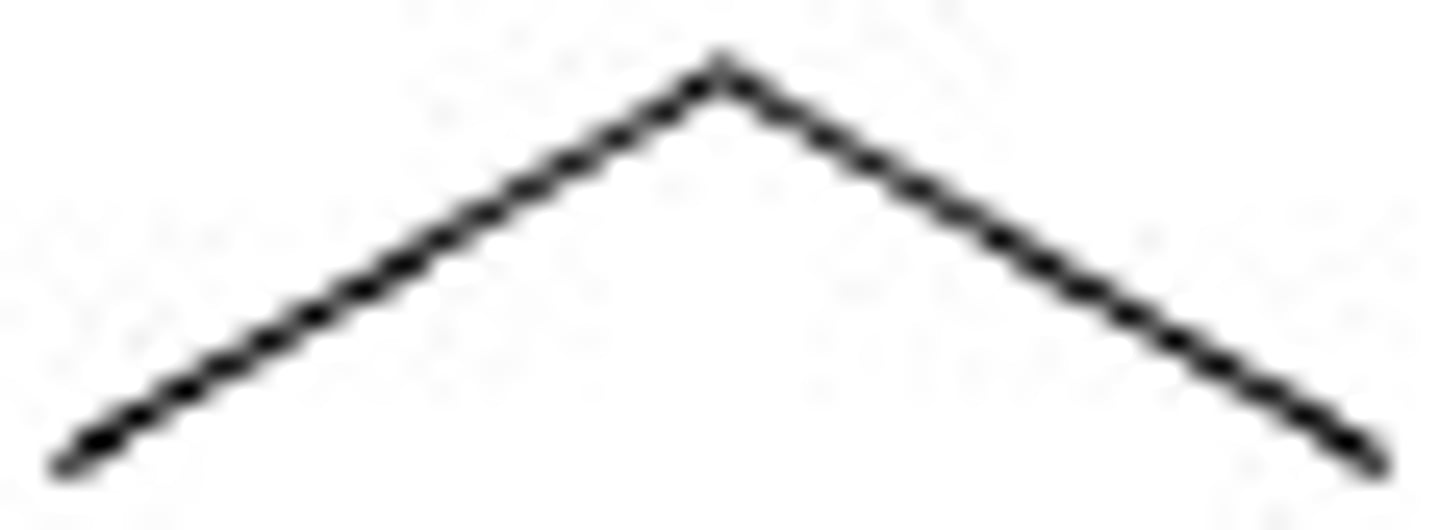
butane

methane
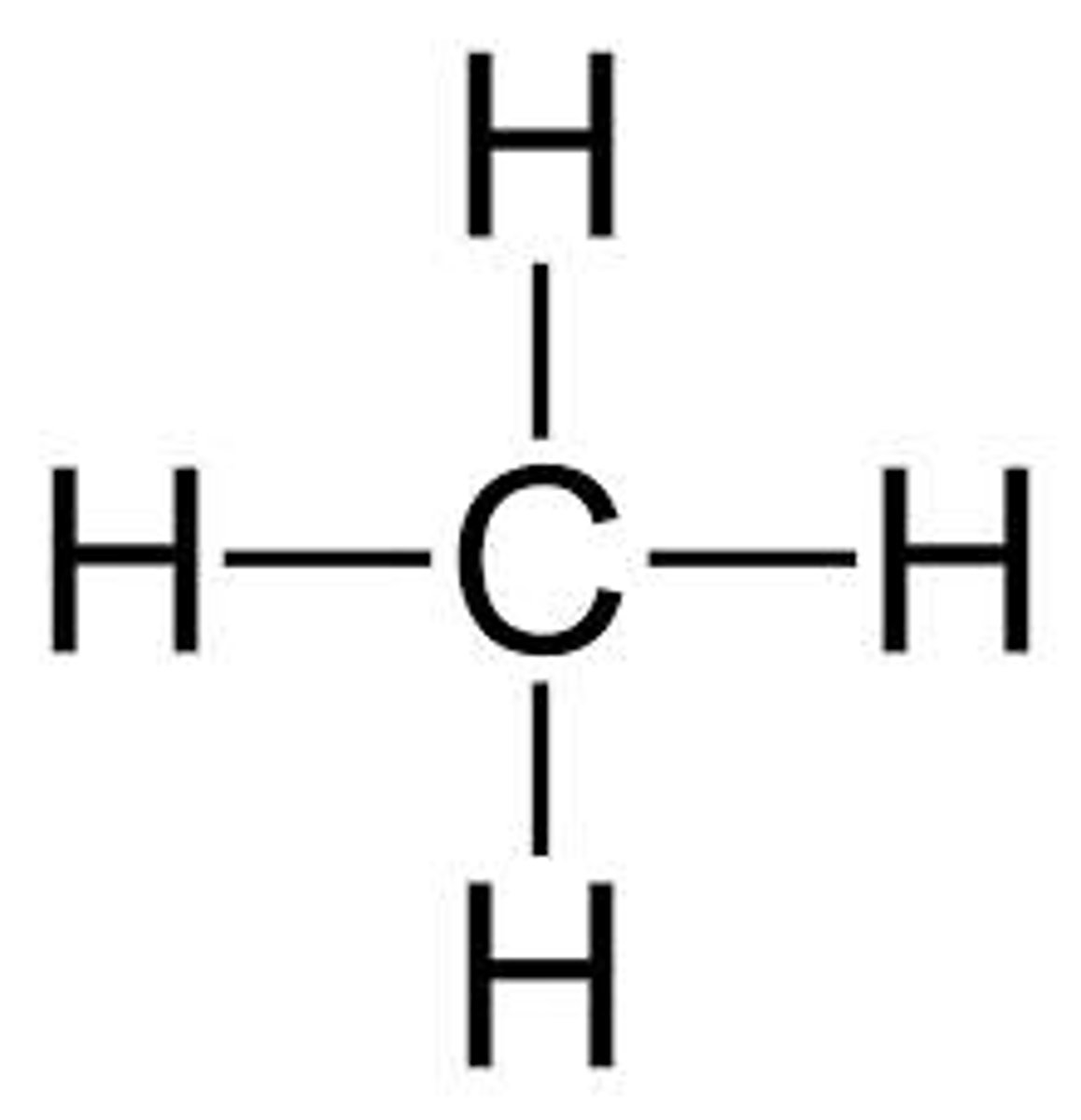
ethane
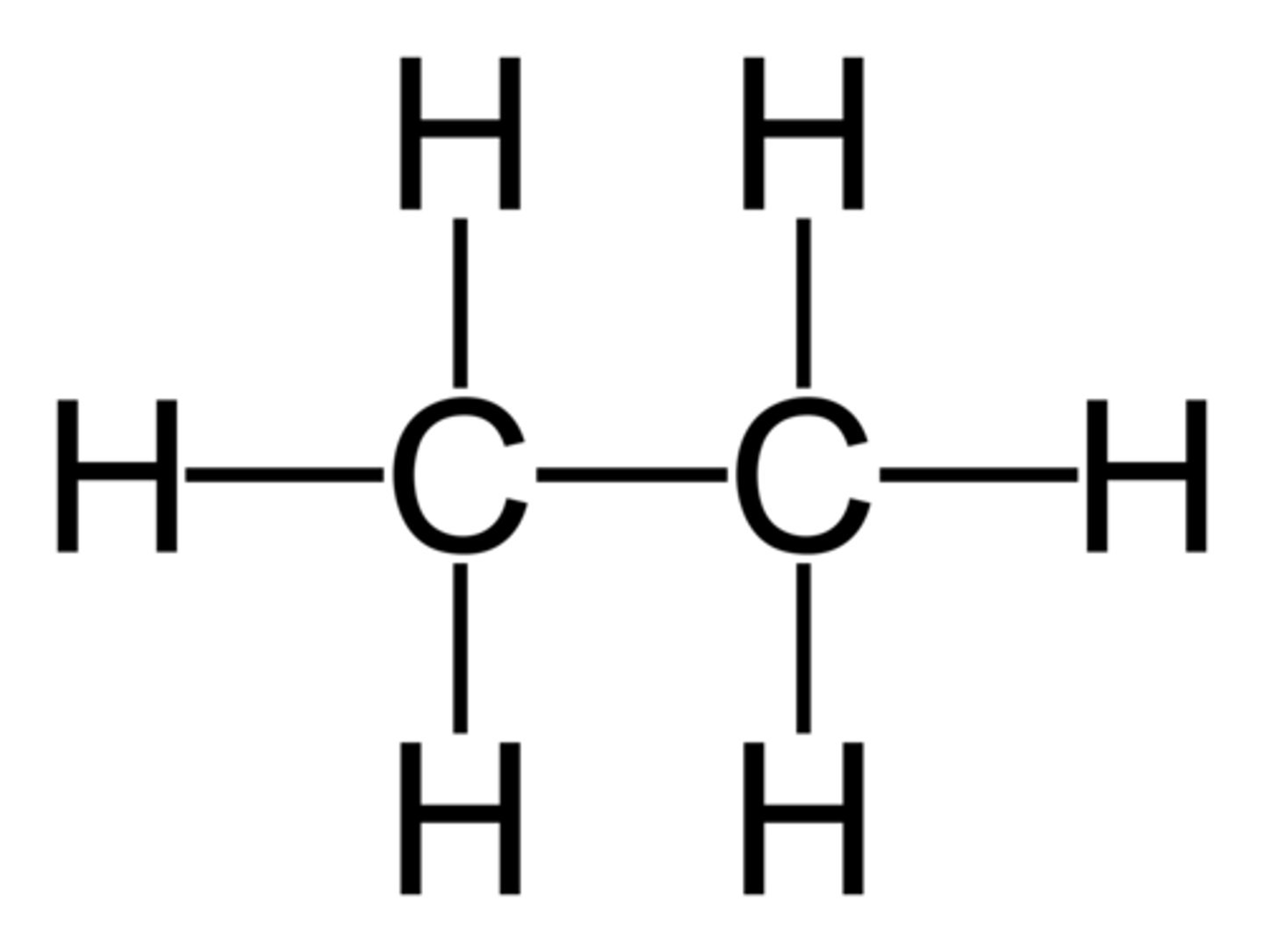
cyclohexene
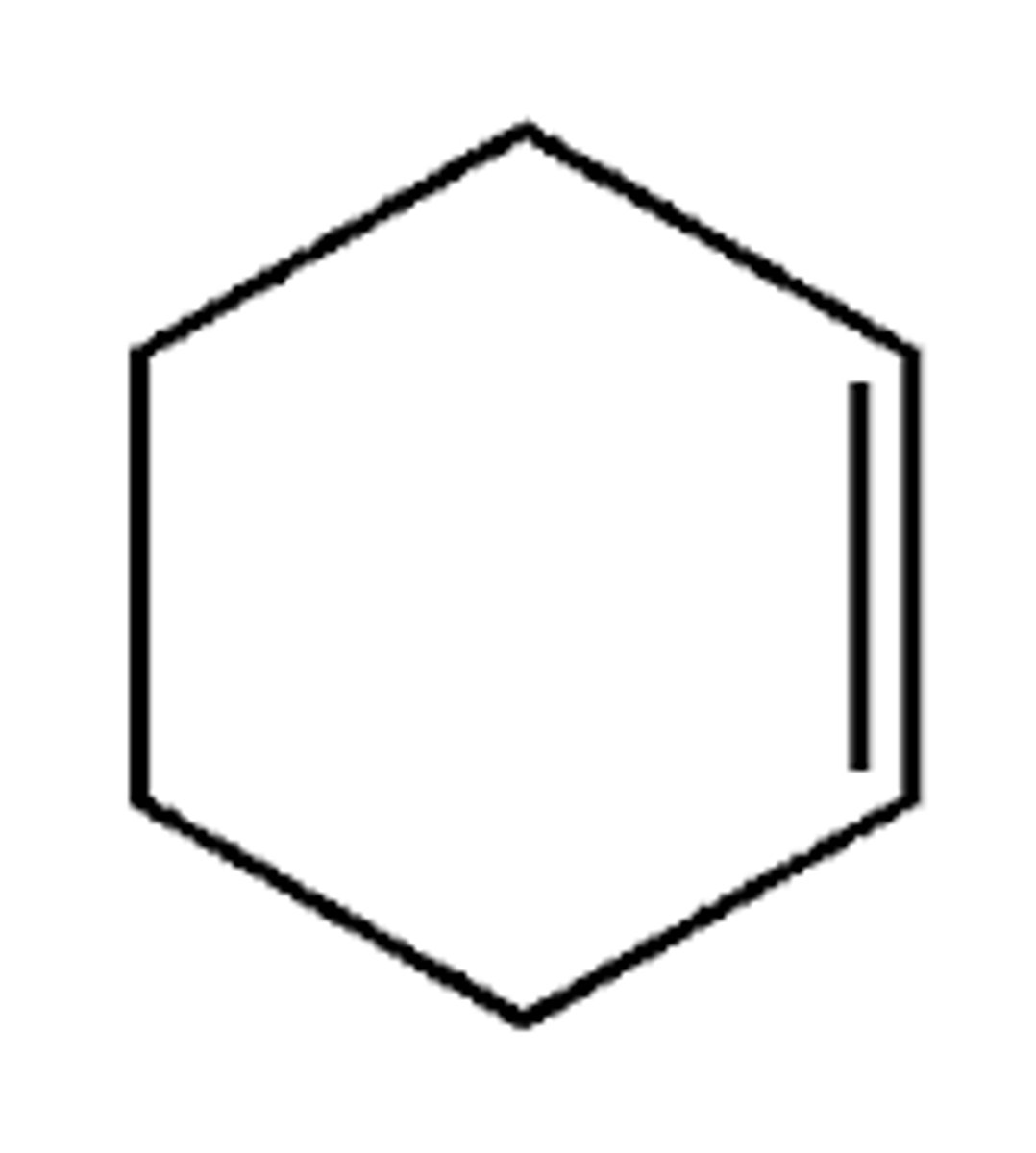
meso
A compound with 2 or more stereocenters centers and an internal plane of symmetry
optically inactive
R
priorities based on atomic number (1,2,3,4) go clockwise
S
priorities based on atomic number (1,2,3,4) go counterclockwise
conformers
due to bond rotation
E
highest priorities based on atomic number are on opposite sides in an alkene
Z
highest priorities based on atomic number are on opposite sides in an alkene