Comprehensive Atomic Theory and Experiments: Dalton, Thomson, Rutherford, Chadwick
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
What is incoreect about Daltons atomic theory
Atoms are NOT all identicle, because isotopeve diffrnet number if neutrons
Who was the first person to describe an atom
Democritus
Atoms
A fundamental unit of matter
What is matter made of
Small particles of atoms (Democritus said this)
Dalton
Studied ratios in which elements combine in chemical reactions.
John Joseph Thomson
Discovered negative particles using a cathode ray tube
Cathode Ray Tube
An experiment, in a tube with metal plates on both ends and an electric charge flowing through the gas inside.
What did the cathode ray tube determine and how
Determined electrons were all atoms, and were negative by showing they'd repel a negative plate (the metal plates)
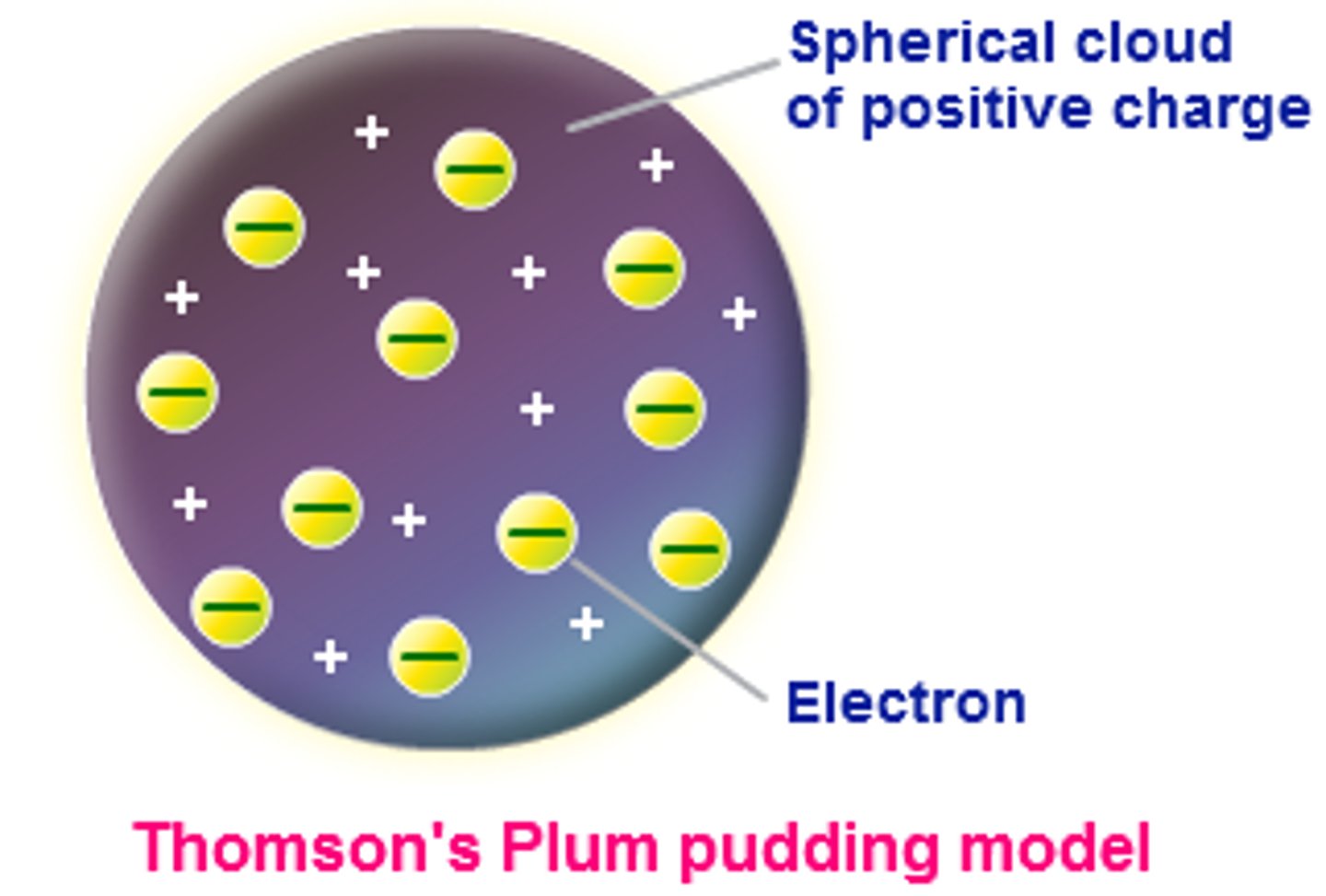
Who made the plum pudding model
Jhon Joseph Thomson
What is the Plum Pudding Model
Positive charge spread over the entire spear, with electrons throughout
Crookes Tube
Put a spinning wheel in a cathode ray tube
What did the Crookes Tube determine.
Determined electrons have mass because they could push the pinwheel
Millikan
Did the oil drop experiment, and determine the value of the charge of the electron?
Millikan
Used Thomson's charge-to-mass ratio for electrons to calculate the mass of a single electron
The Oil Drop experiment
A tube with a metal plate, and an oil drop with electrons on it.
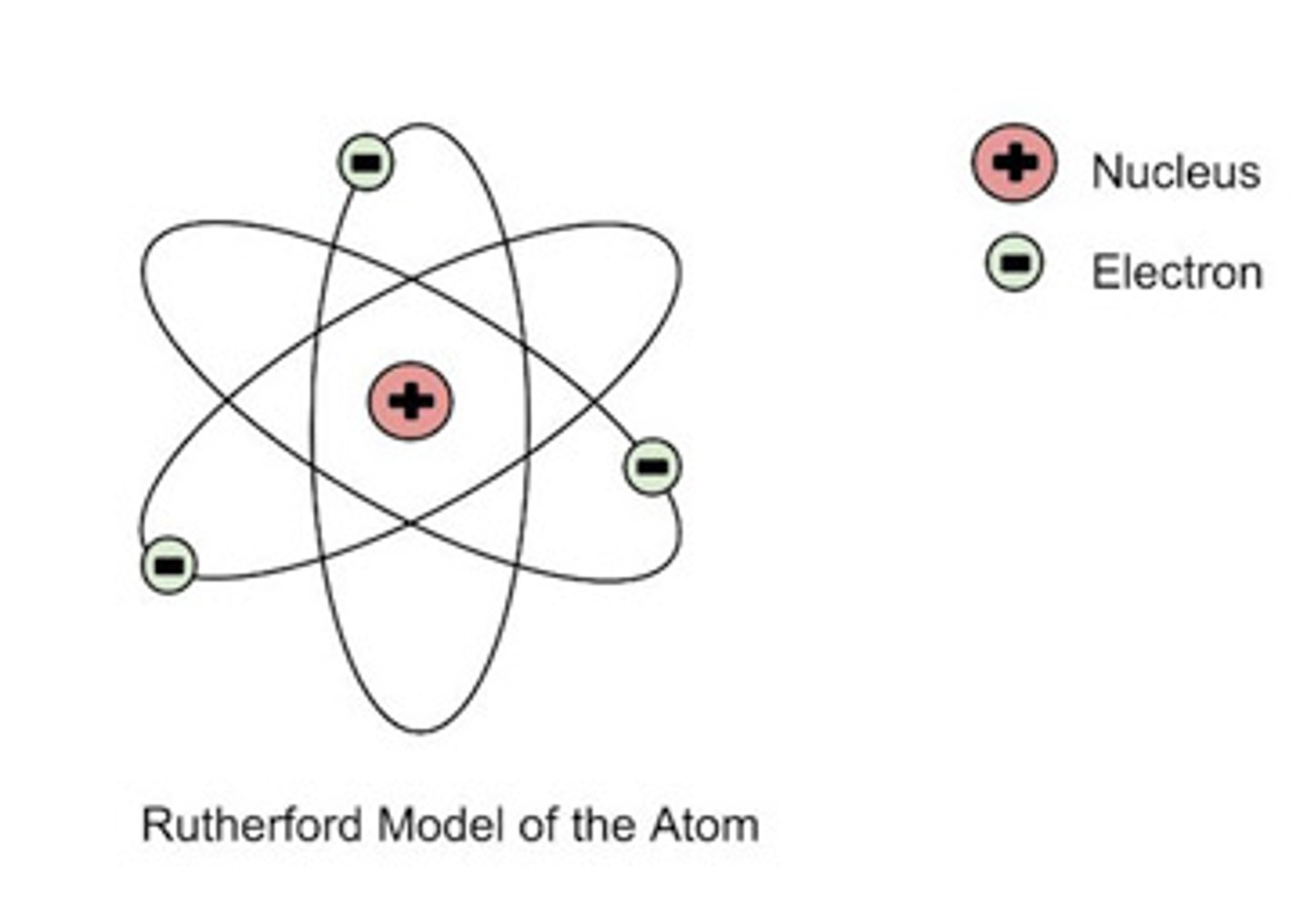
RUTHERFORD
Did the GOLD FOIL EXPERIMENT in order to support the Plum Pudding Model of the atom
Gold Foil Experiment
Gold Foil, tried to put alpha particles through the foil. Instead SOME of the particles bounced off
What did the Gold Foil Experiment determine
concluded that the nucleus must be very small compared to the entire atom
What is a alpha particle
positively charged particles made of 2 protons and 2 neutrons
CHADWICK
Compared the number of protons and mass of hydrogen and helium and realized there was potentially missing mass
Hydrogen Atom
1 proton, 1 electron, Mass: 1 amu
Helium atom
2 protons, 2 electrons, Mass 4 amu
What did Chadwick Devise
an experiment hitting lithium, beryllium, and boron with alpha particles to release a neutral subatomic particle, the neutron
What is the Mass of a proton approximately equal to
mass of a neutron (neutron slightly heavier)
How much heavier is a proton then electron
A proton is about 1840x heavier
What charge does a neutron have
no charge
The numerical charges of a proton and electron are perfectly opposite of each other
A=
mass number=number of protons + nuetrons
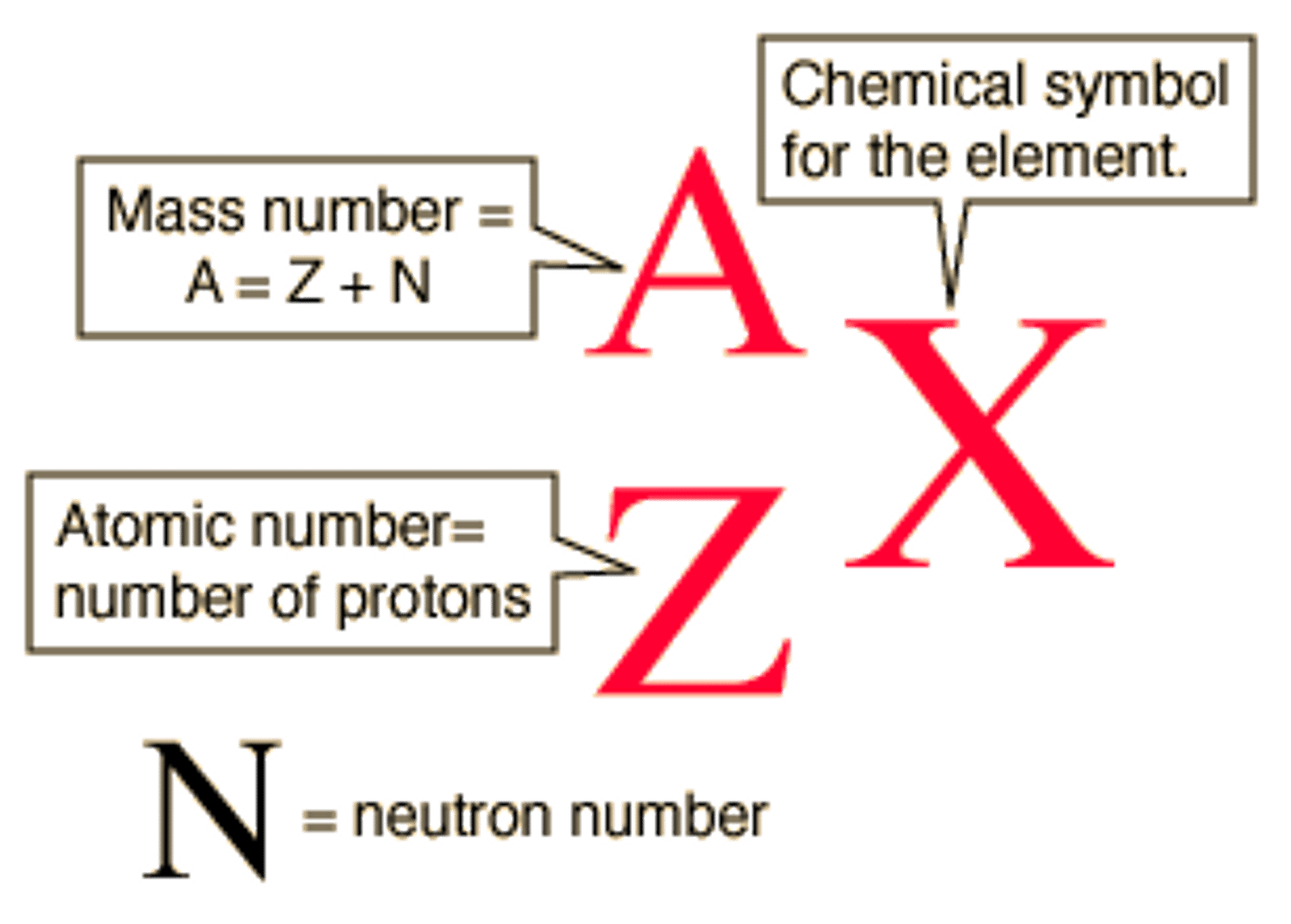
X=
Element Symbols
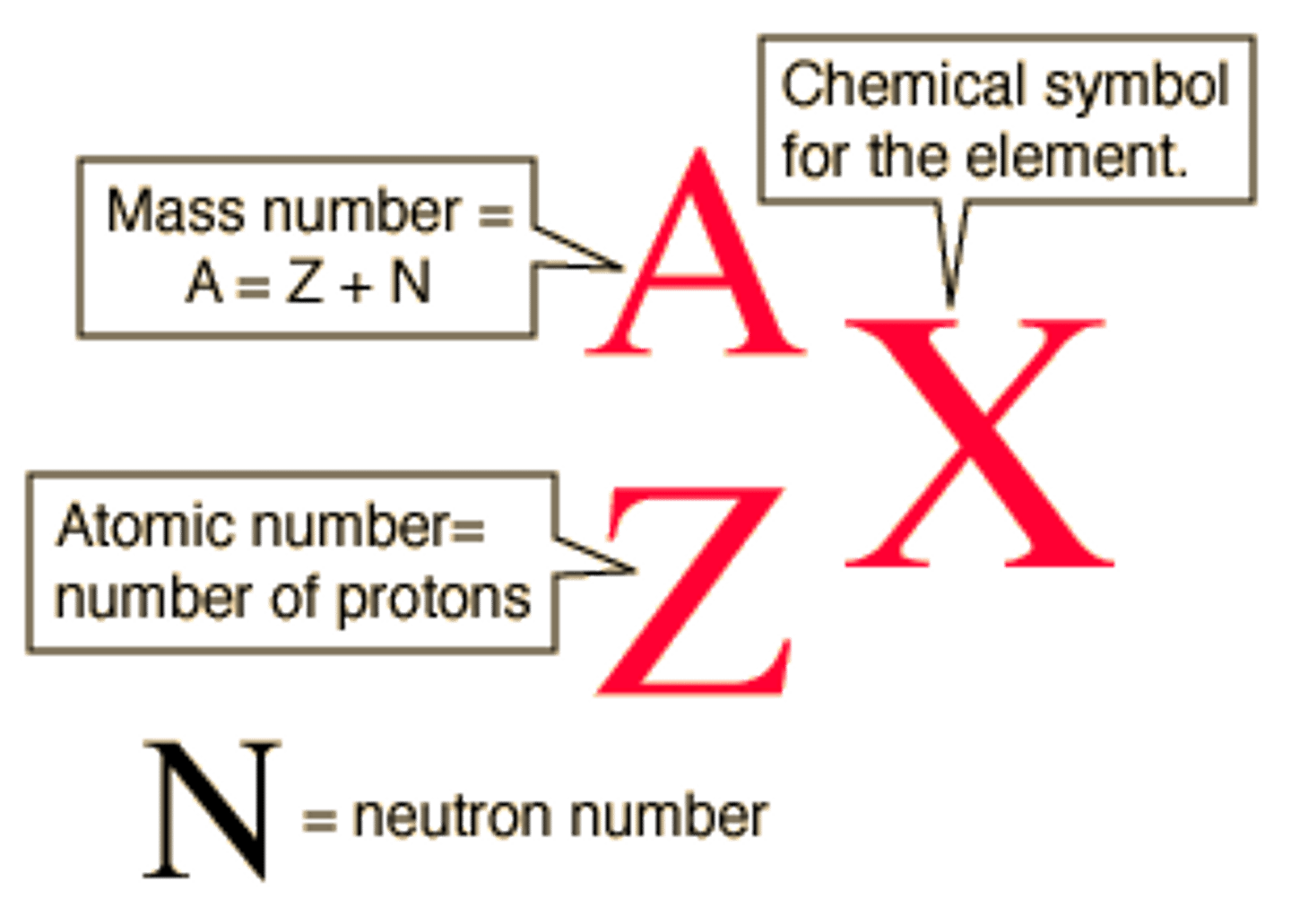
Z=
Atomic Number=Number of protons
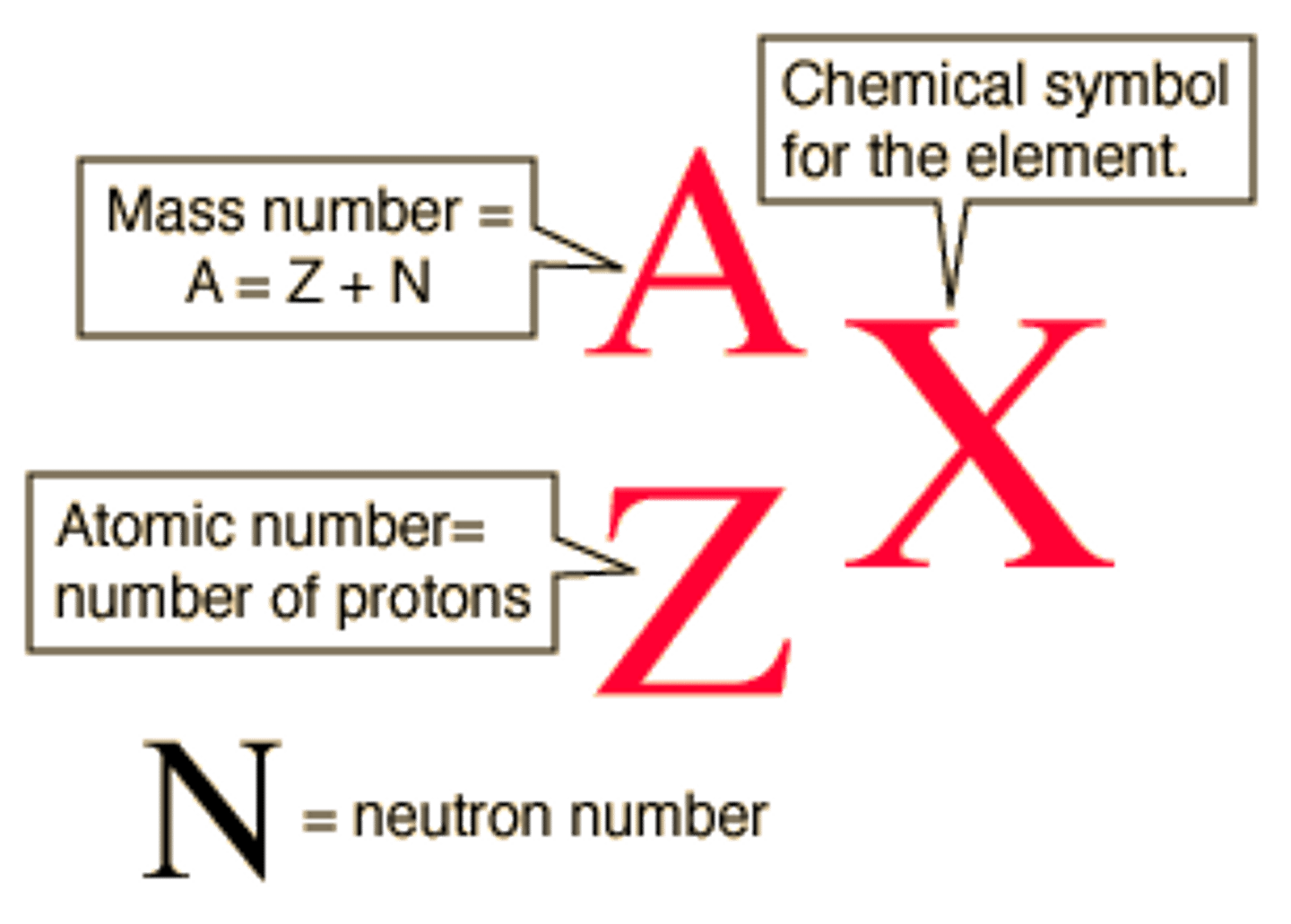
Isotopes
Atoms of the same element with different numbers of neutrons
What does A-Z=
Neutrons
Average Isotopic Mass:
Atomic Mass x (Percent Abundance/100)
Average isotopic Mass, measure unit
g/mol
Rutherford's Model of the Atom
NUCLEUS IS EXTREMELY SMALL
Are atoms indestructible
They are NOT