P4 : Atomic Structure
1/79
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
What is the radius of an atom?
1 × 10-10m
Where is the mass of the atom concentrated?
In the centre where the nucleus is
What 2 particles does the nucleus contain?
Protons and neutrons
What are the 3 subatomic particles called and what is their overall charge?
Neutrons → 0
Protons → +1
Electrons → -1
Where are electrons located in an atom?
On energy levels
What is the relationship between energy levels and their energy in regards to distance from the nucleus?
The further the energy levels are from the nucleus, the higher energy they have
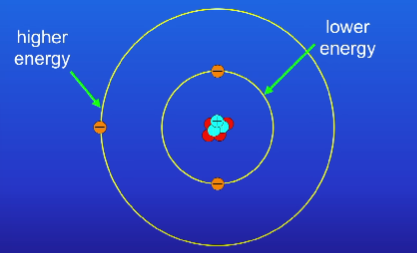
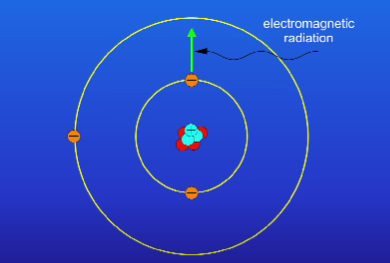
What happens when an electron absorbs electromagnetic radiation?
It can move from a lower energy level to a higher energy level
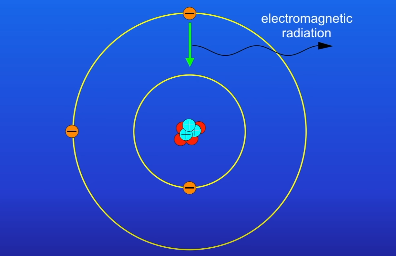
What happens to the electron when the atom emits electromagnetic radiation?
It returns back to the lower energy level
How can electrons change energy levels?
By emitting or absorbing electromagnetic radiation
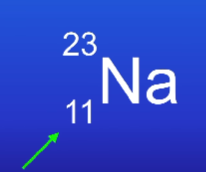
What is this number called and what does it mean?
Atomic number → number of protons
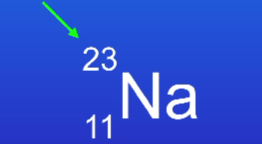
What is this number called and what does it mean?
Mass number → number of protons and neutrons
What is an isotope?
An element which has a different mass number (different number of neutrons)
What is an ion?
When an atom has an unbalanced number of electrons (has either gained or lost electrons)
What were atoms thought to be before the discovery of electrons?
Tiny spheres that could not be divided
What did the discovery of the electron lead to?
The plum pudding model
Explain the plum pudding model
A ball of positively charge with negative electrons embedded into it
Who, when and how was the plum pudding model showed to be wrong?
Earnest Rutherford
1909
Alpha particle scattering experiment
Explain the alpha particle scattering experiment (3)
Positively charged alpha particles were thrown at an extremely thin sheet of gold
From the plum pudding model the particles were expected to pass straight through the sheet of gold or be only slightly deflected
However even though most of them did go through, more were deflected and some came right back
What was the conclusion of the alpha particle scattering experiment + what new model was created?
That the mass of an atom was concentrated at the centre (nucleus) and that the nucleus was charged
The nuclear model
What did Niels Bohr suggest?
That electrons orbit the nucleus in energy levels at specific distances
What did James Chadwick prove?
The existence of neutrons in the nucleus
What is the difference with the plum pudding model and the nuclear model?
The plum pudding model is a ball of positive charge whereas the nuclear model has a positively charged nucleus in the middle
The plum pudding model has electrons embedded into the positively charged ball whereas the nuclear model has electrons orbiting the positively charged nucleus in energy levels
Explain radioactive decay (2)
Some isotopes have an unstable nucleus
To become stable the nucleus gives out radiation
What type of process is radioactive decay?
Random
What is activity in terms of radioactive decay + what is it measured in?
Activity is the rate at which a source of unstable nuclei decays
Activity is measured in becquerel (Bq)
What is becquerel (Bq) of activity equal to?
1 decay per second
What is the count-rate?
Number of decays recorded each second by a detector (e.g. Geiger-Muller tube).
What are the 4 types of nuclear radiation?
Alpha (α)
Beta (β)
Gamma (γ)
Neutron (n)
What is an alpha (α) particle?
A helium nucleus emitted from the nucleus
2 protons
2 neutrons
What is a beta (β) particle?
A high speed electron released from the nucleus
When is a beta (β) particle released from the nucleus?
A beta particle is formed inside the nucleus when a neutron changes into a proton and releases a electron
What is a gamma (γ) ray?
A type of electromagnetic radiation released by the nucleus
What is the range in air for Alpha (α) particles, Beta (β) particles and Gamma (γ) rays?
Alpha (α) → only a few centimetres (lowest range in air)
Beta (β) → a few meters (medium range in air)
Gamma (γ) → long distances (highest range in air)
What is the penetrating power for Alpha (α) particles, Beta (β) particles and Gamma (γ) rays + what are they absorbed by?
Alpha (α) → low ~ a single sheet of paper
Beta (β) → medium ~ sheet of aluminium
Gamma (γ) → high ~ thick sheets of lead or meters of concrete
What is ionising power?
When radiation collides with atoms, that can cause the atoms to lose electrons and form ions
What is the ionising power for Alpha (α) particles, Beta (β) particles and Gamma (γ) rays?
Alpha (α) → strongly ionising
Beta (β) → moderately ionising
Gamma (γ) → weakly ionising
What do nuclear equation show?
Radioactive decay by using elements
What form are nuclear equations written in?
atom before decay → atom after decay + radiation emitted
When an atom emits an alpha particle what happens to its mass number and atomic number?
Mass number decreases by 4
Atomic number decreases by 2
What is alpha decay represented as?
A helium nucleus


What would be the nuclear equation for alpha decay of Uranium?

When an atom emits a beta particle what happens to its mass number and atomic number?
Mass number stays the same
Atomic number increases by 1
What is a beta particle represented as?
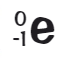

What would be the nuclear equation for beta decay of Carbon?

What do gamma rays get rid of + what does this mean in terms of mass number and atomic number?
Get rid of excess energy
Means no change is mass number and atomic number
What is half life?
The time it takes for the number of radioactive nuclei in an isotope to halve
What does it mean when a source has a short-half life? (3)
Activity falls quickly because the nuclei are very unstable and decay quickly
Sources with a short half life are dangerous because they emit high amounts of radiation at the start
But they quickly become safe
What does it mean when a source has a long half-life? (3)
Activity falls slowly because most of the nuclei don’t decay for a long time
It releases small amounts of radiation for a long time
This is dangerous because nearby areas are exposed to radiation of millions of years
What is irradiation?
The process of exposing an object to nuclear radiation.
Does an irradiated object become radioactive?
No
What 3 ways can reduce the effects of radiation?
Keeping sources in a lead-lined box
Barrier methods or being in a different room
Using remote controlled arms when handling a source
What is contamination?
is when unwanted radioactive isotopes end up on other materials
What can contamination lead to?
Radiation which is harmful
What 2 ways can reduce the effects of contamination?
Gloves and tongs
Protective suits
What type of radiation is the most dangerous outside of the body and why?
Beta and gamma
Because beta and gamma can penetrate the body and get to delicate organs
What type of radiation is the most dangerous inside of the body and why?
Alpha
Because it is highly ionising
What are the risks to using radiation? (3)
Radiation can enter living cells and ionise atoms leading to tissue damage
Lower doses do minor damage without killing cells : however can lead to the rise of mutant cells which divide uncontrollably ; this is cancer
Higher doses kill cells completely causing radiation sickness (vomiting, tiredness + hair loss)
What are the 2 ways nuclear radiation is used in medicine?
Exploring internal organs
Controlling or destroying unwanted tissue
Why would a radioactive tracer be used to explore internal organs? (2)
To check if an organ is functioning normally (thyroid and iodine-123)
To see if a cancer has developed (killing cancer with gamma rays)
Why are gamma rays taken into the body? (4)
Lowly ionising → least harm body tissue
Highly penetrating → radiation passes out of body
Short half life → radioactivity inside the patient quickly disappears
Can be detected by a tracer (beta radiation can be detected too)
Why do people still use tracers even though they could potentially cause cancer? (2)
Risk of cancer via tracer is low
Tracers detect life-threatening conditions
(benefits outweigh the risks)
Why do people undergo radiotherapy even though there are many side risks?
It gets rid of cancer entirely (benefits outweigh the risks)
What is background radiation?
Low level radiation around us all the time
What are the 2 types of natural of background radiation?
Rocks (granite)
Cosmic rays
What are the 2 types of man-made of background radiation?
Fallout from nuclear weapon testing
Nuclear accidents
What 2 things increase how exposed you are to background radiation + give examples?
Location (Cornwall)
Occupation (cabin crew + airline pilot)
What is radiation does measured in?
In sierverts (SV)
1 Sv = 1000 mSv (millisievert)
What are the 2 types of nuclear reactions?
Nuclear fission
Nuclear fusion
What is nuclear fission?
The splitting up of a large unstable nucleus
What does nuclear fission release + how?
Releases energy from large unstable atoms by splitting them into smaller atoms
What does the nucleus have to do before splitting + what does that lead to?
Absorb a neutron which leads it to becoming unstable
What is the product of nuclear fission? (4)
Gamma rays
2 smaller nuclei
2 or 3 neutron
Energy
Why is nuclear fission a chain reaction?
Because the neutrons released after the initial unstable nuclei splits leads to other nuclei to absorb a nucleus and become unstable and split leading to a chain reaction
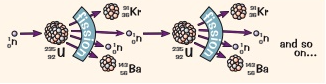
Where is the chain reaction of nuclear fission controlled?
In a nuclear reactor
How is the chain reaction of nuclear fission controlled?
By lowering control rods into the nuclear reactor which absorb neutrons which slow down the chain reactions and keep them under controlled
What happens if the chain reaction of nuclear fission isn’t controlled?
Lead to lots of energy being release as an explosion (this is how nuclear weapons work)
What is nuclear fusion?
The joining of two light nuclei to form a heavier nucleus / 2 light nuclei collide at high speed and fuse together to create a larger heavier nucleus (a hydrogen nucleus fuses together to produce a helium nucleus)
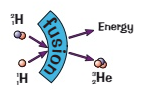
What are the products of nuclear fusion?
A larger heavier nucleus
Energy
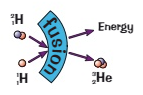
Which one releases more energy, nuclear fission or nuclear fusion?
Nuclear fission
Why haven’t scientist been able to do nuclear fission?
The temperatures and pressures needed for fusion are very high which means that fusion reactors are really hard and expensive to build