Experiment to measure the specific latent heat of fusion of ice
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
13 Terms
Draw a diagram of this Experiment
…
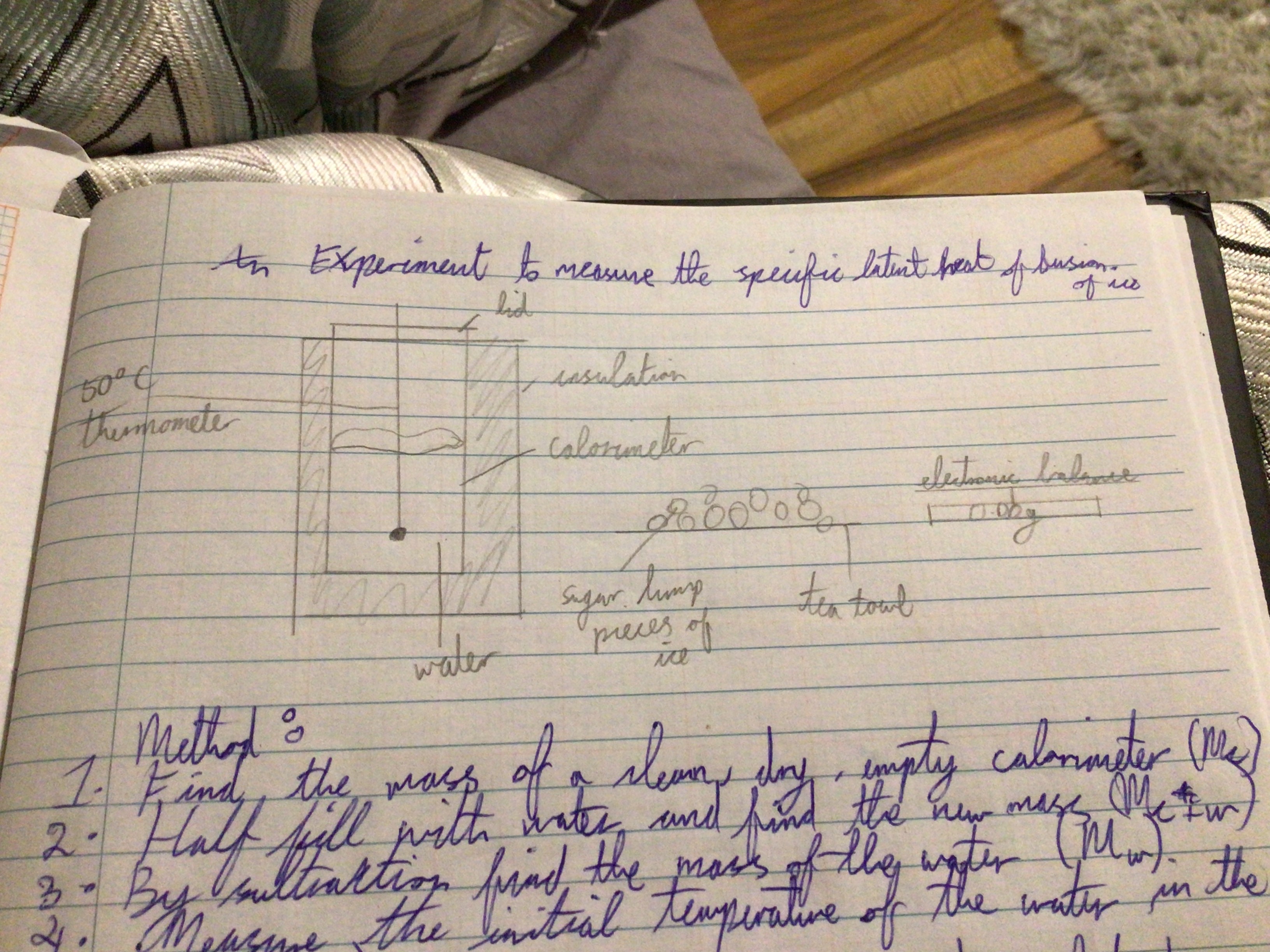
Step 1
Find the mass of a clean dry empty calorimeter (Mc)
Step 2
Half fill with water and find the new mass (Mc + w)
Step 3
By subtraction find the mass of the water (Mw)
Step 4
Measure the initial temperature of water in the can (θi)
Step 5
Set up apparatus as shown and add crushed dry ice until the temperature drops by at least 10 degrees
Step 6
Measure the final temperature (θf)
Step 7
Find the mass of the water+ calorimeter + melted ice (Mc + w + i)
Step 8
By subtraction find the mass of the ice (Mc+w+i -Mc+w) (Mi)
Step 9
Calculate the specific latent heat of fusion of ice from: (Mi)(l) + (Mi)(Cw)(Δθi) = (Mw)(Cw) (Δθw) + (Mc)(Cc)(Δθc)
How to get Δθi?
θf-0
How to get Δθc and Δθw?
θi - θf
What are we looking for in the equation?
l